Anopheles (Cellia) rampae Harbach & Somboon
|
publication ID |
https://doi.org/ 10.5281/zenodo.277125 |
|
DOI |
https://doi.org/10.5281/zenodo.5695415 |
|
persistent identifier |
https://treatment.plazi.org/id/03DF8795-FF9C-A549-FF34-0D9DFAD3206F |
|
treatment provided by |
Plazi |
|
scientific name |
Anopheles (Cellia) rampae Harbach & Somboon |
| status |
sp. nov. |
Anopheles (Cellia) rampae Harbach & Somboon View in CoL , n. sp.
Anopheles maculatus View in CoL form K of Baimai et al., 1993: 116, 117, 119–121 ( Thailand; metaphase karyotype); Harbach, 2004: 548 (notes); Rattanarithikul et al., 2005: 29 ( Thailand; species list); Ma et al., 2006: 274, 275, 277, 278 (ITS2 rDNA, phylogenetic relationships); Rattanarithikul et al., 2006: 27, 32, 45, 77, 125 ( Thailand; Ƥ L keys); Walton et al., 2007: 93, 94, 96– 100 ( Cambodia, Thailand, Vietnam; ITS2 rDNA); Manguin et al., 2008: 496, 497 ( Thailand; review); Somboon et al., 2008: 1317 –1321 ( Thailand; crossmating); Thongwat, 2008 ( Thailand; genetics, morphology); Thongwat et al., 2008: 194–201 ( Thailand; crossmating).
Anopheles maculatus View in CoL species K of Baimai, 1989: 147, 152, 157, 159 ( Thailand); World Health Organization, 2007: 5, 49, 53– 55 ( Thailand; review); Morgan et al., 2009: 591, 292, 294–296, 298 ( Cambodia, Thailand, Vietnam; mtDNA, rDNA, phylogenetic relationships).
Diagnosis. Members of the Maculatus Group are medium-sized mosquitoes that lack upper proepisternal setae, have speckled legs and hindtarsomere 5 entirely pale-scaled. Based on the studies of Rattanarithikul & Green (1987) and Rattanarithikul et al. (2006), the presence of pale scales on most or all of abdominal terga II–VII and vein R2 shorter than vein R2+3 distinguish the adult females of An. rampae from those of An. pseudowillmori . Despite overall similarity, clusters of dark scales on the posterolateral corners of abdominal terga II–VII and the presence of two dark spots on vein R2+3 of at least one wing (or the presector dark spot the same length on vein R as on the costa and subcosta if only one spot is present) readily distinguish An. rampae females from An dravidicus , An. maculatus and An. willmori . Females of An. rampae are usually distinguished from those of An. notanandai by having the median pale spot on veins M1, M2 and M3+4 more than twice as long as the dark spot on either side and the furcation of vein R2+3 within the proximal half of the preapical dark spot on vein R1. Females of An. rampae and An. sawadwongporni are indistinguishable but their eggs are usually distinguished by the greater length and incomplete frill in the former species. Larvae of the Maculatus Group normally have seta 6-III with 20 or more branches. The length of the basal stem of seta 4-M is the only character that distinguishes larvae of An. rampae from those of An. dravidicus , An. maculatus , An. pseudowillmori and An. willmori . The stem of this seta is no longer than four times its width in An. rampae and five or more times its width in the latter four species. The larvae of An. rampae , An. notanandai and An. sawadwongporni are indistinguishable.
The unique metaphase karyotype of An. rampae readily distinguishes this species from the other members of the Maculatus Group: chromosome X2 is metacentric, one arm is entirely heterochromatic whereas the opposite arm consists of a euchromatic portion and centromeric heterochromatin of approximately equal length; chromosome X3 is submetacentric, the short arm consists of a euchromatic portion and a large block of centromeric heterochromatin similar to that of the X2 and the heterochromatic long arm is larger than that of the X2; chromosome Y1 is acrocentric or a small submetacentric and Y2 is very large and submetacentric ( Baimai et al., 1993).
Sequence data for the ITS2 region of ribosomal DNA and the COII and ND5 genes of mitochondrial DNA distinguish An. rampae from the other species of the Maculatus Group (except An. notanandai Rattanarithikul & Green which has not been sequenced) ( Ma et al., 2006; Walton et al., 2007; Morgan et al., 2009; as form K).
Female. Head: Proboscis length 1.78–1.88 mm, 1.10–1.29 length of forefemur; maxillary palpus length 1.63– 1.83 mm, 0.88–0.97 length of proboscis; palpomeres 2 and 3 black-scaled except at apices, palpomere 3 infrequently with dorsomesal patch of pale scales, preapical black band 0.40–0.77 length of subapical white band and 0.32–0.56 length of apical white band; subapical white band 0.57–1.00 length of apical white band. Thorax: Integument dark brown; central area of scutum with narrow white spatulate scales, scales on scutal fossa shorter and broader; scutellum with similar scales. Wing ( Table 1): Preapical dark spot on costa 1.20–2.30 (mean 1.76) length of preapical pale spot; presector dark spot on vein R 0.71–1.15 (mean 0.91) length of corresponding dark spot on costa; vein R2 short, 1.09–1.77 length of vein R2+3; R2+3 with 2 dark spots on at least one wing (85.5%), distal spot near furcation rarely absent, the 2 spots occasionally joined to form a single large spot; vein R4+5 occasionally with median dark spot (on one wing in 8.1% of specimens; on 2 wings in 3.2% of specimens); vein M1 with median pale spot, 0.75–2.3 (mean 1.37) length of dark spot on either side; vein M2 with median pale spot, 0.62– 2.60 (mean 1.46) length of dark spot on either side; vein M3+4 with long median pale spot, 1.25–2.67 (mean 1.83) length of dark spot on either side; vein 1A with short to long median dark spot, 0.23–0.80 (mean 0.49) length of pale spot on either side, dark fringe spots usually present before apex of vein R1 and between apices of veins R2 and R3. Legs: Anterior surface of forecoxa with patch of pale and dark scales at base; femora and tibiae with scattered pale spots and narrow pale patch and/or fringe at apex, ventral surface of forefemur with indefinite stripe of pale scales apically, mid- and hindfemora with indefinite stripe of pale scales ventrally at base; tarsi with pale bands and spots, foretarsomere 1 with 3–7 posterodorsal pale spots, foretarsomere 2 sometimes with a single pale spot in median dark band; midtarsomere 1 with 3–7 posterodorsal pale spots, midtarsomeres 1–3 with narrow pale spot dorsally at apex, midtarsomeres 2 and 3 without median pale spots, midtarsomeres 3 and 4 occasionally with posterodorsal pale spot at base; hindtarsomere 1 with 6–10 posterodorsal pale spots, hindtarsomere 2 sometimes with pale spot in median dark band. Abdomen: Integument dark brown, covered with dark setae; terga II-IV sparsely or densely covered with pale spatulate scales posteriorly, posterolateral corners of terga III and IV usually with few black spatulate scales; terga V–VIII largely covered with pale spatulate scales, also with conspicuous patches of black spatulate scales on posterolateral corners; sterna IV–VII with few scattered pale spatulate scales (occasionally on sternum III as well) and median patch of black spatulate scales on posterior margins; sternum VIII with pale falcate and/or spatulate scales laterally.
Characteristics Females (n = 62) Males (n = 61)
One wing Both wings One wing Both wings (%) (%) (%) (%) Presector dark spot on vein R as long as presector dark spots
on subcosta and costa 54.8 40.3 70.5 54.1 Vein R2 +3 with two dark spots 85.5 64.5 86.9 73.8 Vein R4 +5 with 3 dark spots 8.1 3.2 11.5 4.9 Vein M1 with median pale spot>2 times length of dark spot
on either side 19.4 8.1 50.8 23.0 Vein M2 with median pale spot>2 times length of dark spot
on either side 72.6 53.2 83.6 63.9 Vein M3+4 with median pale spot>2 times length of dark
spot on either side 72.6 50.0 68.9 52.5 Furcation of vein R2+3 within proximal 0.5 of preapical dark
spot on vein R1 48.4 29.0 0 0 Male. Like female except as follows. Head: Proboscis length 2.08–2.18 mm, 1.31–1.50 length of forefemur; maxillary palpus length 2.08– 2.03 mm, 0.95–1.02 length of proboscis. Wing: Characteristics compared with those of female in Table 1. Legs: Foretarsomeres 2 and 3 with apical pale patch or band only. Abdomen: Posterolateral corners of terga III and VII occasionally with few black spatulate scales; tergum VIII (ventral in position) with variable amount of pale scaling, with median patch of black spatulate scales posteriorly; sternum VIII (dorsal in position) largely pale-scaled. Genitalia ( Fig. 1 View FIGURE 1 C–E): Gonocoxite with scales on lateral surface, with 4 or 5 slender and lanceolate parabasal setae; gonostylus longer than gonocoxite, with prominent subapical tergolateral setae and row of minute setae along sternolateral margin; gonostylar claw short, pigmented. Claspette with tergolateral club formed of fused stems and 1 or 2 slender apical setae longer than club. Aedeagus narrow, apex usually with 4 flattened leaflets on either side, distal leaflets serrate on outer margin. Proctiger membranous, lightly sclerotised laterally.
Egg. Length 0.49–0.54 mm, width 0.12–0.15 mm; deck usually complete; frill incomplete in middle on both sides, distance between anterior and posterior frills 0.20–0.33 length of deck; ratio of width to length of part of deck enclosed by anterior frill 0.14–0.27, ratio of width between floats to length of part of deck between anterior and posterior frills 0.23–0.62, ratio of width to length of part of deck enclosed by posterior frill 0.16–0.31; float with 14–20 ridges, confluent with median part of deck (part without frill).
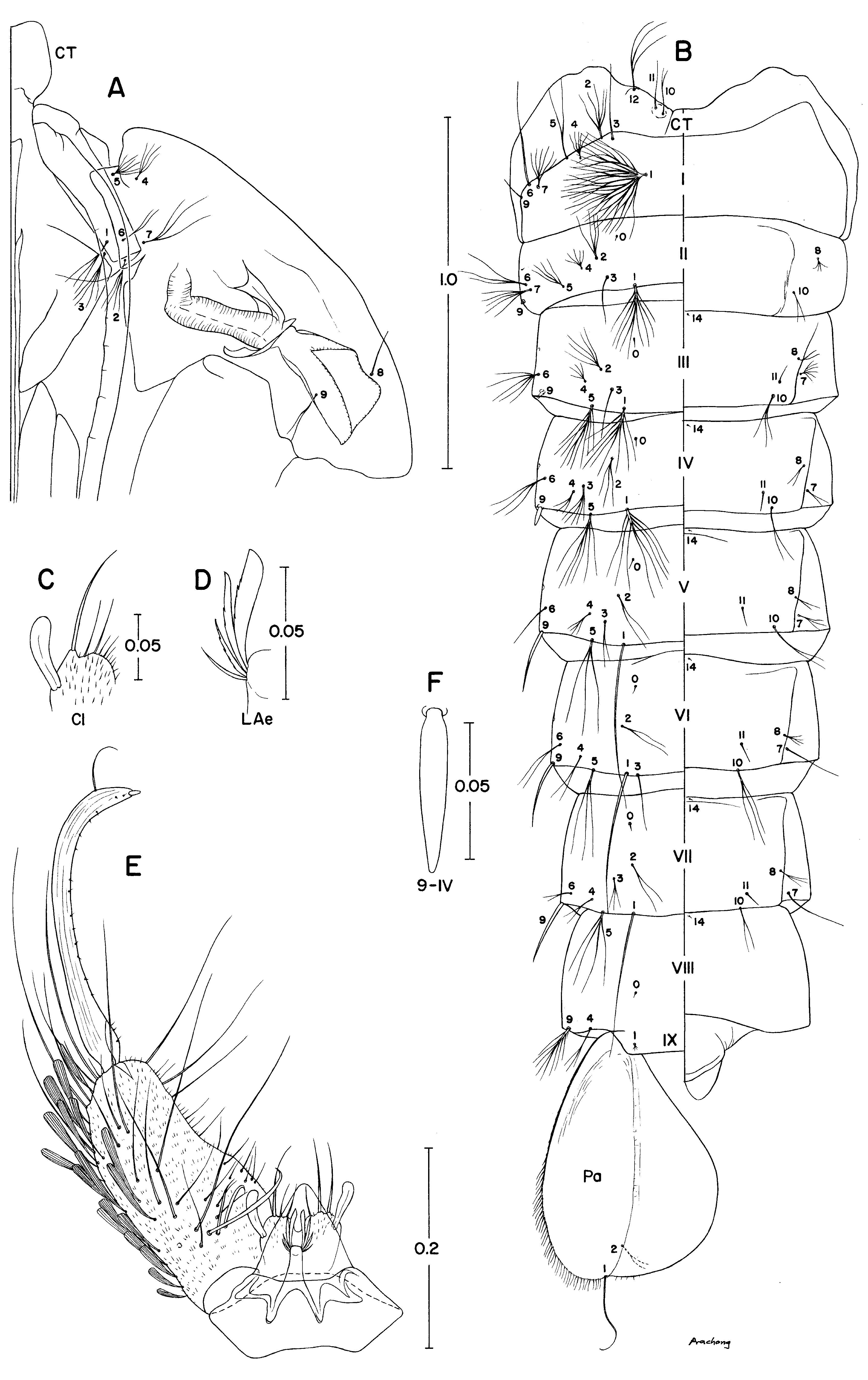
Pupa ( Fig.1 View FIGURE 1 A,B). Character and positions of setae as figured; range and modal number of branches in Table 2. Cephalothorax: Trumpet length 0.35–0.43 mm, width 0.08–0.11 mm, index 3.64–4.00. Abdomen: Seta 9-II,III very short, blunt; 9-IV–VII long, sharply pointed; ratio of length of seta 9-III / 9-IV 0.30–0.55 (mean 0.37), 9-IV / 9-V 0.26–0.38 (mean 0.30); 9-VIII with 9–19(13) branches. Paddle: Seta 1-P generally shorter, 0.31–0.44 length of paddle.
Seta Cephalothorax Abdominal segments Paddle no. CT I II III IV V VI VII VIII IX Pa 0 – – 1,2(1) 1 1 1 1,2(1) 1 1 – – 1 2–4(3) nc 4–9(5) 4–10(6) 2–8(3) 1,2(1) 1,2(1) 1,2(1) – 3,4(4) 1 2 2–4(3) 3–8(4) 3–7(4) 3–7(5) 2–4(3) 2–4(3) 2,3(2) 1–3(2) – – 1–3(2) 3 2–4(3) 1,2(1) 1–3(1) 1–3(2) 1–6(4) 1–3(2) 1–3(1) 1–3(2) – – – 4 2–6(3) 2–6(3) 1–7(4) 1–6(3) 1–4(3) 1–5(3) 1–3(1) 1–3(1) 1–3(2) – – 5 4–8(5) 1–4(3) 2–6(4) 5–11(7) 3–10(5) 2–7(4) 3–6(4) 3–6(4) – – – 6 2–4(3) 1–5(3) 3–6(3) 2–8(3) 1–4(3) 1–4(1) 1–3(1) 1–3(1) – – – 7 3,4(3) 2–7(3) 3–6(3) 2–6(4) 1–6(3) 1–5(2) 1–3(1) 1,2(1) – – – 8 1–3(1) – 3–5(4) 1–5(3) 1–3(1) 1,2(1) 1–4(2) 1–4(3) – – – 9 2–4(3) 1,2(1) 1 1 1 1 1 1 9–19 (13) – – 10 1,2(2) – 1,2(1) 1–4(2) 1,2(1) 1–3(2) 1–3(2) 1–4(2) – – – 11 1–5 (3) – – 1,2(1) 1 1,2(1) 1 1,2(1) – – – 12 1–5 (3) – – – – – – – – – – 14 – – – 1 1 1 1 1 1 – –
nc = not counted.
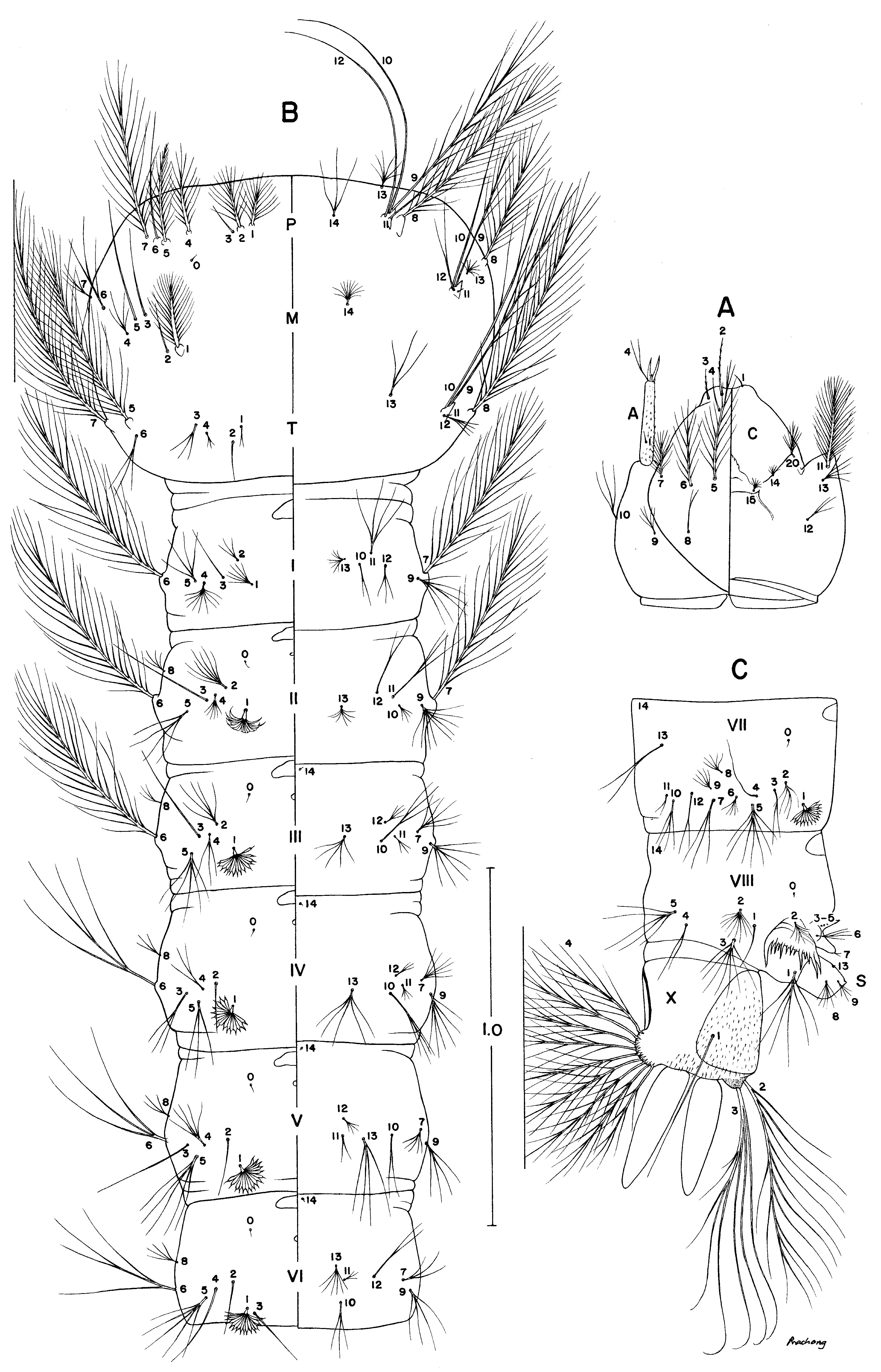
Larva, fourth-instar ( Fig. 2 View FIGURE 2 ). Character and placement of setae as figured; range and modal number of branches in Table 3 View TABLE 3 . Head: Seta 4-A with 2,3(2) branches; seta 2-C single, with 5–12 short lateral aciculae. Thorax: Seta 4-M with 3–5(4) branches, width of basal stem 0.20–0.40 (mean 0.28) of its length; seta 3-T with 1–4(4) branches. Abdomen: Seta 1-I not palmate, with 4–7(5) branches; seta II–VII palmate; seta 1-II with 8–12(10) weakly developed leaflets, 1-III with 13–18(16) fully developed leaflets, filaments usually <0.25 length of blades; leaflets of seta 1-IV–V each with relatively long filament, 0.19–0.38 length of blade; seta 4-I with 4–10(7) branches; seta 5-VII with 7–13(9) branches. Pecten plate with 4–6 long and 6–10 short spines.
DNA sequence. Ma et al. (2006) and Walton et al. (2007) reported that the nucleotide sequence of the ITS2 region of ribosomal DNA in An. rampae (as form K) is unique among members of the Maculatus Group ( An. notanandai not included), differing by 3.7% ( Walton et al., 2007) from An. sawadwongporni Rattanarithikul & Green , the most closely related species. More recently, Morgan et al. (2009) also found a strong sister relationship between An. rampae (as form K) and An. sawadwongporni based on a Bayesian analysis of the mitochondrial COII and ND5 genes.
Etymology. This species is named in honor of Dr Rampa Rattanarithikul (formerly of the Armed Forces Research Institution of Medical Sciences, Bangkok) for her many important contributions to our knowledge of mosquitoes in Southeast Asia, especially her studies of the Maculatus Group ( Rattanarithikul & Green, 1987; Rattanarithikul & Harbach, 1991) that provided the taxonomic foundation for further studies of this medically important group of insects.
Systematics. The correlation of morphological differences with paracentric inversions of polytene chromosomes and heterochromatin variation in mitotic chromosomes allowed Rattanarithikul & Green (1987) and Rattanarithikul & Harbach (1991) to give formal Latin names to eight members of the Maculatus Group. Polytene chromosomes have not been studied in the ninth member of the Group, i.e. An. rampae , but as noted above the unique metaphase karyotype readily distinguishes this form from the other species. In particular, the X and Y chromosomes of An. rampae are different from those of An. notanandai and An. sawadwongporni , the most morphologically similar species.
Mitotic karyotypes from brain ganglia of fourth-instar larvae of An. rampae (form K) and An. maculatus forms B and E are generally similar, consisting of three types of X chromosomes (small submetacentric X1, metacentric X2 and large submetacentric X3) and two types of Y chromosomes (acrocentric or small submetacentric Y1 and large submetacentric Y2) ( Green et al., 1985; Baimai et al., 1993). Previously, Baimai et al. (1993) reported that X3, Y1 and Y2 occur in form B; X1, X3 and Y1 occur in form E; and X2, X3 and Y2 occur in form K. However, Thongwat (2008) found that X2 and Y2 occur in all three forms.
Seta Head Thorax Abdominal segments * Shown in Fig. 2 View FIGURE 2 with fewer than actual branches; nc = not counted.
Crossing experiments involving An. rampae (as An. maculatus form K), An. maculatus forms B and E, An. sawadwongporni , An. dravidicus , An. pseudowillmori and An. willmori ( Somboon et al., 2008; Thongwat et al., 2008) revealed that An. rampae is genetically distinct with varying degrees of genetic incompatibility with the other species. In all crosses, hybrid males were sterile or partially sterile with abnormal and inactive spermatozoa or atrophied testes and accessory glands. In some crosses, male embryos failed to develop or emerge from the eggs. High mortality of larval and/or pupal stages was also observed. One or both ovaries were normal or atrophied in hybrid females. Backcrosses resulted in more severe incompatibility in both sexes. The polytene chromosomes of the ovarian nurse cells in F1 hybrid females exhibited approximately 70% to complete asynapsis of all chromosome arms.
Bionomics. Anopheles rampae has been found in sympatry with An. maculatus , An. pseudowillmori and An. sawadwongporni . The immature stages have been found in small rock and sand pools exposed to sunlight, often with green algae, along the Mekong River and in hilly forested areas, about 100–400 m above sea level. Adult females start biting shortly after sunset (18:00–20:00); they are primarily zoophilic but will sometimes bite humans. Whether or not An. rampae plays a role in human malaria transmission is unknown.
Distribution. Anopheles rampae (as An. maculatus form K and species K) has been found in localities in northeastern Thailand (Ubon Ratchathani Province – Baimai et al., 1993; Walton et al., 2007; Somboon et al., 2008; Thongwat et al., 2008; Morgan et al., 2009), northern Cambodia (Ratanakiri and Vihear Provinces – Walton et al., 2007; Morgan et al., 2009) and central Vietnam (Quang Binh Province – Walton et al., 2007; Morgan et al., 2009). Anopheles rampae has also been found in other areas of northeastern Thailand (Chaiyaphum, Loei, Mukdahan, Nakhon Phanom, Nong Bua Lam Phu, Nong Khai and Phetchabun Provinces) and in Laos (Khammouan Province) (R. Rattanarithikul, unpublished observations). Specimens that form part of the Type series (see below) were collected in Udon Thani Province, also in northeastern Thailand.
Type series. One hundred and fifty-nine specimens (33 Ƥ, 23 3, 47 Le, 52 Pe, 4 L) derived from four cytotyped progeny broods: UDN 7(3), UDN 7(9), UB 500(14) and UB 501(2). Holotype, Ƥ (UDN 7(9)-12), with Le and Pe on microscope slide, offspring of female collected as follows, THAILAND: Udon Thani Province, Nong Bua Lam Phu (17º 13′ N, 102º 27′ E), 20 Oct 1986, biting human, coll. Udom. Paratypes, same data as holotype: 16 Ƥ LePe [UDN 7(9)-1, -10, -13 through -16, -18, -20, -21; UDN 7(3)-2, -3, -5, -12 through -15]; 3 Ƥ Pe [UDN 7(3)-1, -16; UDN 7(9)-19)]; 4 Ƥ [UDN 7(9)-M [mother], UDN 7(9), UDN 7(9)-17; UDN 7(3)-17]; 13 3 LePe [UDN 7(9)-2, -4 through -9; UDN 7(3)-6 through -11; 1 3 Pe (UDN 7(3)-100]; 1 3 [UDN 7(9)]. Paratypes, offspring of female collected as follows, THAILAND: Ubon Ratchathani Province, Na Chaluai (14º 25′ N, 105º 14′ E), 7 Oct 1987, biting human, coll. Udom’s team: 8 Ƥ [UB 501(2)-28, -29, -32, -34, -35; UB 500(14)-21, -22, - 100]; 2 Ƥ [UB 501(2)-27; UB 500(14)]; 7 3 LePe [UB 501(2)-26, -30, -31, -36 through -39]; 1 Pe [UB 501(2)-27]; 4 L [UB 501(2)-A, -B, -C, -D].The type series is deposited in the Natural History Museum ( BMNH), London.
TABLE 3. Range (mode) of numbers of branches for fourth-instar larval setae of Anopheles rampae.
| no. C P | M | T I | II |
|---|---|---|---|
| 0 1 1 | – | – – | 1 |
| 1 1 15–23(19) | 26–46(40) | 1–3(3) 4–7(5) | 8–12(10) |
| 2 1 14–22(19) | 1–3(2) | 1 3–6(3) | 5–7(6) |
| 3 1 1 | 1 | 1–4(4) 1,2(1) | 1 |
| 4 1 13–19(16) | 3–5(4) | 2–5(4) 4–10(7) | 5–11(7) |
| 5 11–22(15) 27–49(40) | 1 | 23–40(30) 4–6(5) | 5–7(6) |
| 6 11–21(19) 1 | 3–6(4) | 2–5(3) 24–32(30) | 21–32(29) |
| 7 17–25(20) 16–24(22) | 2–4(3) | 21–36(33) 24–31(27) | 24–36(30) |
| 8 1,2(1) 27–39(35) | 14–22(20) | 29–42(35) – | 2–4(3) |
| 9 1–6(4) 11–16(14) | 8–15(11) | 12–22(19) 5–7(5) | 7–9(8) |
| 10 1–4(3) 1 | 1 | 11–19(13)* 1–3(2) | 3–5(3) |
| 11 34–41(39) 2–5(3) | 1,2(1) | 1 3–5(4) | 1–3(2) |
| 12 3–6(3) 1 | 1,2(2) | 2–6(4) 3–5(4) | 2–4(3) |
| 13 4–7(6) 2–7(5) | 5–11(6) | 3,4(3) 4–8(7) | 5–8(7) |
| 14 nc 3–5(4) | 7–11(10) | – – | – |
| 15 nc – | – | – – | – |
| continued. | |||
| Seta Abdominal segments | |||
| no. III IV | V | VI VII VIII | X |
| 0 1 1 | 1 | 1 1 1 | – |
| 1 13–18(16) 12–18(15) | 13–17(14) | 11–16(13) 12–16(13) 1–3(2) | 1 |
| 2 4–7(5) 1,2(1) | 1,2(1) | 1–3(1) 4–7(5) 8–13(11) | 13–24(20) |
| 3 1 2–5(3) | 1–3(1) | 1,2(1) 2–5(3) 6–10(9) | 7–14(11) |
| 4 3–7(4) 3,4(3) | 2–6(4) | 1,2(1) 1,2(1) 2,3(3) | – |
| 5 4–7(5) 3–5(4) | 5–9(6) | 5–10(8) 7–13(9) 5–8(6) | – |
| 6 18–28(22) 3–5(5) | 3–5(4) | 3–5(5) 4–7(4) – | – |
| 7 3–7(5) 4–7(5) | 3–6(5) | 2–6(4) 3–7(4) 1-S, | 5–10(6) |
| 8 1–4(3) 2,3(2) | 2–4(3) | 2–4(3) 3–6(6) 2-S, | 4–12(8) |
| 9 5–9(7) 4–6(5) | 4–6(5) | 4–7(6) 3–9(6) 6-S, | 2–4(3) |
| 10 2–4(3) 2–4(3) | 1–3(2) | 2–5(4) 4–10(6) 7-S, | 1–3(2) |
| 11 2–5(3) 2–5(3) | 2,3(2) | 2–4(3) 2–4(3) 8-S, | 4–7(5) |
| 12 2–4(2) 3–5(3) | 2–4(3) | 2,3(2) 2–4(2) 9-S, | 5–9(7) |
| 13 4–7(5) 4–6(5) | 3–6(5) | 5–11(8) 2–6(4) – | – |
| 14 1 1 | 1 | 1 1 1 | – |
| 15 – – | – | – – – | – |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
|
SubGenus |
Cellia |
Anopheles (Cellia) rampae Harbach & Somboon
| Somboon, Pradya, Thongwat, Damrongpan & Harbach, Ralph E. 2011 |
Anopheles maculatus
| Manguin 2008: 496 |
| Somboon 2008: 1317 |
| Walton 2007: 93 |
| Ma 2006: 274 |
| Rattanarithikul 2006: 27 |
| Rattanarithikul 2005: 29 |
| Harbach 2004: 548 |
| Baimai 1993: 116 |