Rossella levis (Kirkpatrick, 1907)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3692.1.6 |
|
publication LSID |
lsid:zoobank.org:pub:E86E41ED-D12B-4E3D-9FA3-25C8B2923183 |
|
DOI |
https://doi.org/10.5281/zenodo.5631276 |
|
persistent identifier |
https://treatment.plazi.org/id/03C387F0-AB1F-FFD8-FF6A-FAF20B48F8B0 |
|
treatment provided by |
Plazi |
|
scientific name |
Rossella levis (Kirkpatrick, 1907) |
| status |
|
Rossella levis (Kirkpatrick, 1907) View in CoL
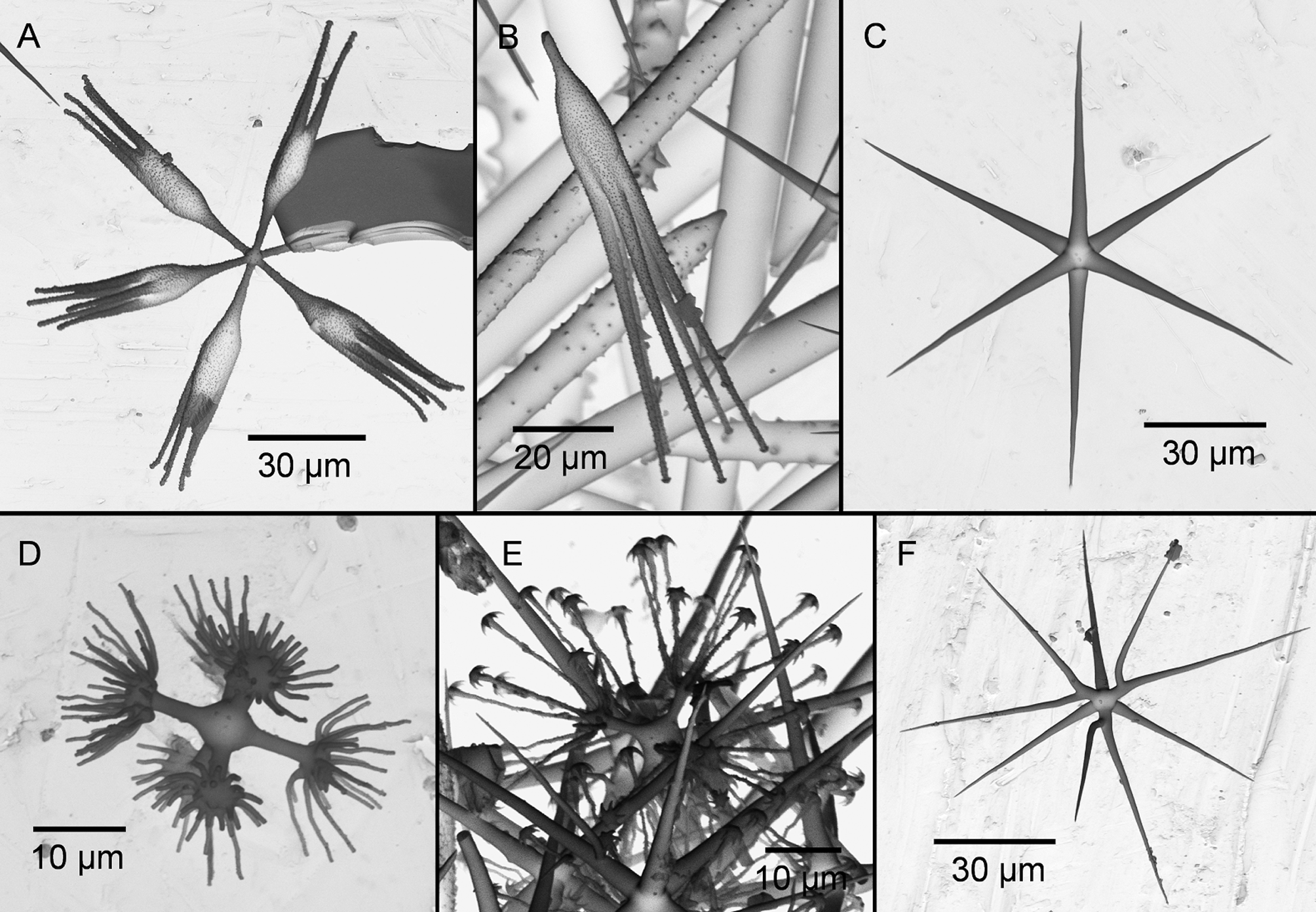
( Figs. 2 View FIGURE 2 D, 5, Tab. 4 View TABLE 4 )
Synonymy:
Aulorossella levis Kirkpatrick, 1907: 17 –19, pl. 2, figs. 2–3, pl. 6, fig. 3. Barthel & Tendal 1994: 101–104, fig. 41 – 42, pl. 11– 12.
Aulorossella pilosa Kirkpatrick, 1907: 16 –17, pl. 2, fig. 1, pl. 6, fig. 2. Topsent 1917: 17–18.
Aulorossella longstaffi Kirkpatrick, 1907: 19 –20, pl. 2, fig. 4, pl. 7, fig. 1.
Aulorossella gaini Topsent, 1916: 164 –165; 1917: 18–19, pl. 5, fig. 2.
Anaulosoma schulzei Kirkpatrick, 1907: 21 –23, pl. 3, fig. 5–6, pl. 5, fig. 2. Burton 1929: 411.
Material examined. 3 specimens from SYSTCO-station 48-1 (11728, 11903, 11904). Other material examined: BMNH 1908.2.5.17.
Description. One specimen (SMF 11728, Fig. 2 View FIGURE 2 D) is relatively large (9 cm in height) and shows the growthform usually considered typical for the species. It has a firm tissue with a relatively low amount of spicules in comparison to other Rossella spp. The inner surface is very smooth. The outer surface bears no velum of protruding pentactins, but numerous, regularly distributed conules of up to 5 mm in height, which in many cases bear spicule tufts solely composed of diactins. These spicule tufts are especially abundant at the basis of the sponge, where the spicules are up to about 10 cm long and form a root-like system, which fixes the sponge to the ground. All rooting spicules originate from distinct and separate spicule tufts, in contrast to the rooting structure present in other Rossella spp., where tufts merge to one compact structure at the sponge base. The terminal osculum is relatively tight and appears contracted. The body is barrel-shaped with a wide inner cavity, which reaches a diameter of 2.5 cm. The body wall is 1 cm thick. The other specimens present are small, juvenile ones. One (SMF 11903) is chosen for detailed description here. The specimen is 2.1 cm high and 1.8 cm wide. The body is vase-shaped, but very rounded. The surface is firm without a velum. Numerous small conules are regularly distributed over the body. They are very flat and mostly bear spicule tufts of about one to four protruding spicules, which reach a length of up to 1.5 cm. These protruding spicules include diactins and pentactins, in a ratio of about 3 to 1. Pentactins occur mostly at the bottom, forming a weak rooting system. All specimens sampled are of white color, both alive and in ethanol.
The species is best characterized by the external appearance lacking a veil of pentactins (raised pentactins only occurring in the bottom part of the sponge), but having long protruding diactins on distinct conules, and by its characteristic calycocomes ( Fig. 5 View FIGURE 5 A–B) and microdiscohexasters ( Fig. 5 View FIGURE 5 D–E). The latter are relatively rare, thus the figure shows, to our knowledge the first SEM-photographs ever published of these spicules from R. levis . They are 45 µm in diameter, have secondary rays of only one length and resemble the shape of the microdiscohexasters of R. antarctica . They have a strong knot-like structure in the middle and weak or absent capitula at the end of the primary rays, where the secondary rays originate. Discs at the end of the secondary rays are well developed and have strong recurved hooks. The secondary rays are weakly granulated. The calycocomes ( Fig. 5 View FIGURE 5 A–B) are 170 µm in diameter, have medium-sized primary rays and middle pieces, each 15 µm long, 2–7 secondary rays, 70 µm long, mostly moderately curved, regularly granulated with small, distinct discs. Oxyhexactins ( Fig. 5 View FIGURE 5 C) of 100 µm in diameter are very numerous and therefore quite characteristic for the species. Oxyhexasters and hemioxyhexasters ( Fig. 5 View FIGURE 5 F), 95 µm, are much rarer.
Remarks. In contrast to most other species described here, the spicule measurements of our R. levis specimens fall well within the dimensions already reported ( Tab. 4 View TABLE 4 ; Barthel & Tendal (1994)).
Burton (1929) synonymized R. levis with R. nuda as a variation with extremely long protruding diactins on extraordinary well developed conules. However, the two species differ significantly in spiculation: R. levis has much smaller calycocomes with more secondary rays and a completely different microdiscohexaster with secondary rays of only one length. In our opinion it represents a well characterised species. Similar microdiscohexasters occur only in R. antarctica , which is distinguished from R. levis by the possession of a well developed veil of free pentactines and smaller principal calycocomes. The outer appearance of R. levis is very similar to R. villosa Burton, 1929 . These species can be readily distinguished by their microdiscohexasters: R. villosa , as reported by Barthel and Tendal (1994), has microdiscohexasters with secondary rays of two different lengths (like R. racovitzae , R. nuda , R. vanhoeffeni and R. fibulata ). On the basis of the microdiscohexasters R. villosa seems to be much closer related to R. racovitzae than to R. levis , as R. racovitzae has almost identical microdiscohexasters and often a similar shape, especially when young. Molecular phylogenetic analyses of several Rossella spp., including one R. levis (SMF 11728), by Vargas et al. (in review) suggest a close relationship between R. levis and R. racovitzae , as opposed to R. antarctica , which seems to be distinguished very clearly from other Rossella spp.
One additional point to be made concerns the practicability of determination: as mentioned above, the characteristic microdiscohexasters are very rare. Still, in most cases of determination, it might be fitting, to just consider the characteristic outer shape, the very abundant oxyhexactins (while only few oxyhexasters are present) and the relatively small calycocomes. This should usually lead to a reliable identification of the species.
S–C: 26 S–C: 14
C: 5 C: 9
TABLE 4. Spicule sizes of Rossella levis (Kirkpatrick, 1907). Values in μm are given as follows: minimum – mean – maximum (number of spicules measured). For comparison, values from Kirkpatrick (1907) and Barthel & Tendal (1994) are given.
| parameter | SMF 11728 | SMF 11903 | Kirkpatrick (1907) Barthel & Tendal (1994) |
|---|---|---|---|
| rough Pentactin | |||
| tangential ray (L) | 100–143.2–180 (34) | 95–163.7–200 (31) | 150 |
| proximal ray (L) rough Hexactin (D) Stauractin ray (L) | 100–117.2–140 (9) 120–117.2–970 (33) | 110–294.3–155 (7) 190–371.5–540 (20) 140–182.9–245 (33) | |
| Oxyhexactin (D) Oxyhexaster (D) | 60–105.3–130 (33) 60–96.6–135 (31) | 80–100.3–160 (38) 80–96.9–120 (13) | 100 55–180 120 |
| Heterodiactin (L) | |||
| Discohexactin (D) Microdiscohexaster (D) Mesodiscohexaster (D) | 42.5–46.8–52.5 (11) | 70 (1) 40–44–47.5 (10) | 40 34–47 96 45–97 |
| Calycocome | |||
| (D) | 120–170–220 (2) | 170–205–250 (6) | 220 130–230 |
| complete ray (L) | 55–105.3–180 (31) | 50–99.8–150 (30) | |
| primary ray (L) middle piece (L) secondary ray (L) | 10–13.8–20 (31) 10–22.6–47.5 (31) 25–65.5–92.5 (31) | 2.5–9.9–15 (30) 5–13.6–28.75 (30) 30–75.1–115 (30) | 14 8–12 9 6–12 |
| number of sec. rays | 3–5.4–7 (31) | 2–4.3–6 (30) | 6–8 4–8 |
| Curvature | S: 0 | S: 7 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |