Calliapagurops charcoti
|
publication ID |
https://doi.org/10.5281/zenodo.199554 |
|
DOI |
https://doi.org/10.5281/zenodo.6209002 |
|
persistent identifier |
https://treatment.plazi.org/id/E320692D-0733-FF98-D68D-2F04FB43F88E |
|
treatment provided by |
Plazi |
|
scientific name |
Calliapagurops charcoti |
| status |
|
Calliapagurops charcoti de Saint Laurent, 1973
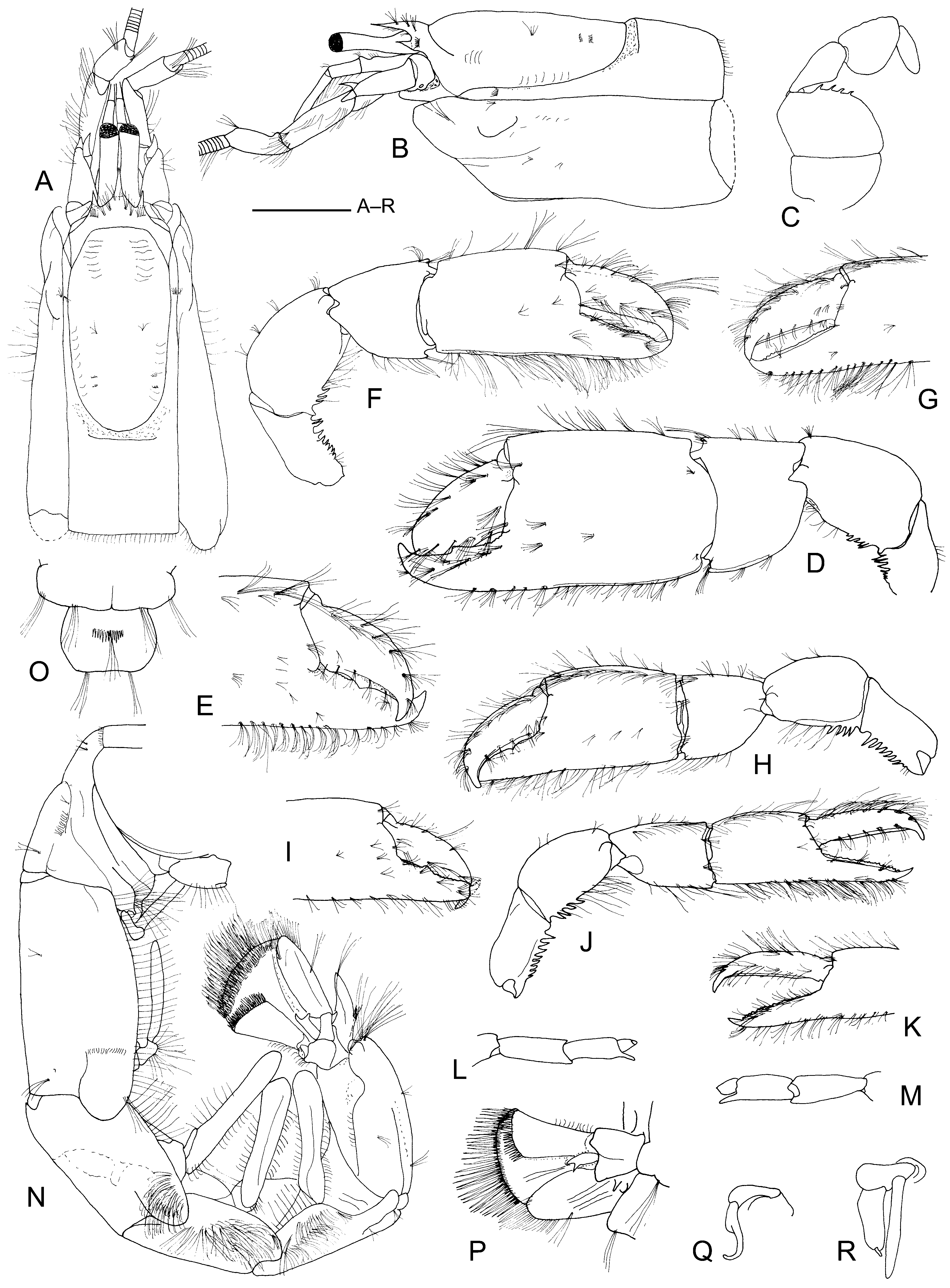
Figs 1–2 View FIGURE 1 View FIGURE 2 .
Calliapagurops charcoti de Saint Laurent, 1973: 515; Manning & Felder, 1991: 771; Sakai, 1999: 8, fig. 1; Tudge et al., 2000: 141; Ngoc-Ho, 2002: 540; 2003: 487, fig. 16.
Material. 1 male (cl 18.3, left fourth pereopod and abdomen missing), Madeira, off Hotel Roca Mar, Caniço de Baixo, 19 m depth, P. Wirtz coll. 6 August 2010, MMF 41170; – 1 female (cl ca 15, posterior carapace damaged, both fifth pereopods and abdomen missing), NHMW 25025; 1 female (tl ca 77, cl ca 17, broken in the middle of cephalothorax, third abdominal somite damaged), NHMW 25026, Madeira, Caniço de Baixo, off Hotel Galomar, 20 m depth, P. Wirtz coll. 27 August 2010.
Additional description. Carapace ca 0.2 times total length. Adult individuals with distinct dorsal oval ( Fig. 2 View FIGURE 2 B). Antennal flagella exceeding in length twice carapace length; flagella with long setae ventrolaterally on both sides of each segment (spacing 210 µm), setae between 3 (distally) and 6 mm (proximally) long, with short (50 µm) setulae. Third maxilliped ( Fig. 2 View FIGURE 2 C) merus with 4 to 6 spines on distal border. First pereopods' coxa with strong, anteriorly curved spine mesially. Chelipeds sexually dimorphic, male major cheliped ( Fig. 2 View FIGURE 2 D) more massive than that of female ( Fig. 2 View FIGURE 2 H), propodus ca 1.4 times length (including fixed finger) and ca 1.5 times height that of female, which is similar in shape but slightly smaller than male minor cheliped. Male minor cheliped with propodus 1.2 times as long and ca 1.5 times as high as that of female. Abdomen ( Fig. 2 View FIGURE 2 N) long, dorsal length ratio (along midline) of first to sixth abdominal somites 1.0: 1.35: 1.0: 0.84: 1.0: 1.0. Telson ( Fig. 2 View FIGURE 2 O) subrectangular, 1.4 times as broad as long, broadest at midlength, posterior border slightly concave. Uropod ( Fig. 2 View FIGURE 2 P) exceeding telson, endopod elongate-ovate, more than twice as long as wide, exopod with distinct dorsal plate. Female first pleopod ( Fig. 2 View FIGURE 2 Q) consisting of two articles of same length, second article with shoulder. Female second pleopod ( Fig. 2 View FIGURE 2 R) biramous, protopod expanded mesially, exopod slender, longer and more slender than endopod, latter with appendix interna.
Colour. Entirely white (female NHMW 25025, and female in Fig. 1 View FIGURE 1 B, C) or white with reddish-brown bands on chelipeds and dorsally on abdominal somites, red antennal peduncles and flagella ( MMF 41170, see Fig. 1 View FIGURE 1 A; female NHMW 25026).
Remarks. Ngoc-Ho (2002, 2003) described the dorsal oval of both Calliapagurops species as faint. The specimens from Madeira, however, have a very pronounced dorsal oval, the anterior depression distinct and deep. This difference seems to depend on the size of the specimens; the male from Madeira has twice the size as the holotype from the Azores. The dorsal oval is usually less distinct also in small specimens of the genera Corallianassa Manning, 1987 and Neocallichirus Sakai, 1988 when compared to larger individuals (PCD, pers. obs.).
Manning & Felder (1991) placed Calliapagurops in the subfamily Callianassinae . Sakai (1999) introduced the monotypic subfamily Calliapaguropinae for this genus. Ngoc-Ho (2002) showed that Calliapagurops is very close to Corallianassa and argued that it is better placed into the subfamily Callichirinae Manning & Felder, 1991.
The behaviour of Calliapagurops observed in Madeira is also similar to that reported for Corallianassa , namely appearing at the burrow opening, catching drifting objects, which are pulled into the burrow (see Dworschak et al., 2006, Kneer, 2008).
One of the unique features of Calliapagurops is the strong antennal peduncles and antennal flagella beset with long setae. The antennae with spread setae are held up into the water column ( Fig 1 View FIGURE 1 B) and are probably used for suspension feeding – which would represent a feeding mode not yet reported for any species of the infraorder Axiidea.
The shallow-water fauna of Madeira is a mixture of species from the temperate Mediterranean-Atlantic region, species with boreal origin and a strong component of tropical species ( Wirtz, 1998). Several recent surveys have revealed numerous new species and new records for this area (e.g. Wirtz, 1998, 1999, 2006). Up to now, no burrowing shrimp (Axiidea and Gebiidea) have yet been found in Madeira. This may be mainly due to their cryptic lifestyle because most species live in deep burrows ( Dworschak, 2000); some probably are night-active and easily overlooked if not specifically targeted. The second author has, however, noted burrow openings likely belonging to this species during numerous dives along the south coast of Madeira Island, usually at depths greater than 30 m.
The finding of Calliapagurops charcoti in shallow waters at Madeira is surprising. The bathymetric range of the species between 20 and more than 200 m is one of the widest ever reported for this group ( Dworschak, 2000). The potential novel feeding mode using the antenna for suspension feeding will be subject of further studies.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Calliapagurops charcoti
| Dworschak, Peter C. & Wirtz, Peter 2010 |
Calliapagurops charcoti
| Ngoc-Ho 2002: 540 |
| Tudge 2000: 141 |
| Sakai 1999: 8 |
| Manning 1991: 771 |
| Saint 1973: 515 |