Cambarellus (Cambarellus) zacapuensis, Pedraza-Lara, Carlos & Doadrio, Ignacio, 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3963.4.5 |
|
publication LSID |
lsid:zoobank.org:pub:4E907734-E5DC-450D-94C2-8A977F6578F6 |
|
DOI |
https://doi.org/10.5281/zenodo.6112582 |
|
persistent identifier |
https://treatment.plazi.org/id/A819CC59-FF81-2013-B09D-FE0EFB3D112B |
|
treatment provided by |
Plazi |
|
scientific name |
Cambarellus (Cambarellus) zacapuensis |
| status |
sp. nov. |
Cambarellus (Cambarellus) zacapuensis View in CoL , new species
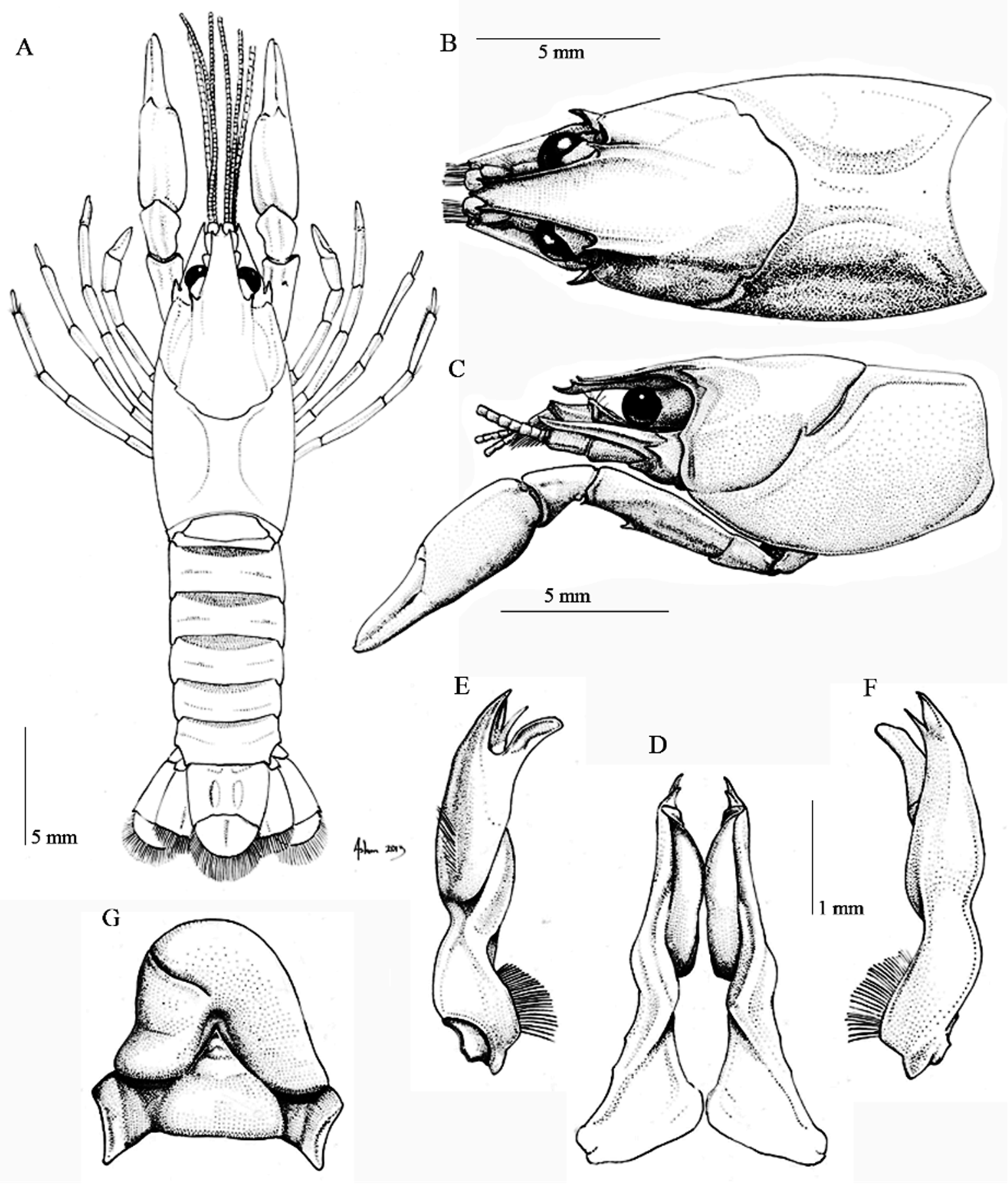
Fig. 4 View FIGURE 4
Diagnosis. Body pigmented, eyes well developed. Rostrum with marginal spines. Acumen length ranging from 9.2–26.7% of RL (x = 19.6%). Caparace wide between 0.45–0.49 times caparace length and from 19.7 to 22.7% (x = 21.2) of TL, without cervical spine. Width of areola 0.48 to 0.52 (x = 0.50) times areola length and 15.6 to 16.4% (x = 16.0) of CL. Suborbital angle from acute to slightly obtuse. Branchiostegal spine absent. Cephalic side of postorbital ridges acute, slightly convergent, ending anteriorly in acute spine. Antennal scale approximately 0.44 times as wide as long, maximum width at about proximal third. Latero-ventral surface of merus of cheliped with one spine. Hooks on ischia of second and third pereiopod monotuberculate and acute, coxae of fourth and fifth pereiopods with prominent cephalo and caudo-mesial bosses. First pleopod of form I male symmetrical, arched caudally at proximal third, with distinct hump on cephalic surface at about midlength, lacking both, cephalic shoulder and subapical setae, but with two rows of simple setae, one composed by of longer setae located along the caudal margin of proximal third and another with shorter ones along mesiocephalic surface of second third; terminal elements consisting of corneous, caudodistally directed mesial process bearing longitudinal groove on its mesial surface and reaching distally well beyond the other elements; central projection corneous, tapering apically and curved caudodistally with tip directed at a 20º angle regarding the axis of appendage; caudal process slender, directed caudodistally at about 30 degrees to shaft and reaching caudally beyond the central projection. Annulus ventralis 1.1 to 1.3 times as broad as long from ventrocaudal view, caudal surface with proximomedian concavity receiving postannular sclerite when moved posteriorly; transverse, undulating sinus situated either sinistrally or dextrally, extending from lateral to distal part of proximomedian concavity and ending on lateral surface of annulus. Postannular sclerite conical, width at base from 1.18 to 1.22 times its height and about two-thirds width of annulus.
Holotypic male, Form I. Cephalotorax ( Fig 4 View FIGURE 4 , A–C) subovate, maximum width almost the same as the cephalotorax height at caudodorsal part of cervical groove (proportion = 0.99). Areola 0.48 times as wide as long, its length 15 % that of CL. Surface of caparace punctuate. Rostrum width 0.56 times of RL; concave in dorsal view, with slender lateral carinae slightly converging anteriorly and ending in short spines. Acumen short, 0.19 times in RL, almost reaching distal end of last segment of antennular peduncle. Subrostral ridge evident along caudal third of rostrum. Postorbital ridge short, terminating cephalically in acute spine. Suborbital angle subacute, with rounded apex. Branchiostegal spine absent, only forming a subacute angle. Cervical spine absent.
Abdomen width 0.88 times cephalotorax width (4.82/ 5.46 mm). Pleura of third to fifth segments rounded. Cephalic lobe of epistome joined to main body, with irregular anterolateral borders and prominent anteromedian projection; main body between epistomal zygoma and cephalic lobe with broad depression; epistomal zygoma arched. Proximal podomere of antennular peduncle with strong ventromesial spine at about midlength. Antennal peduncle with well defined spine on distolateral surface of basis and very small one on ventral surface of ischium. Antennal scale 0.44 times as wide as long; mesial margin of lamellar area broadly rounded; distolateral spine reaching slightly beyond ultimate podomere of antennular peduncle.
Third maxilliped length exceeding beyond tip of rostrum; slightly overreaching antennal peduncle; mesial half of ischium and merus with broad row of stiff simple setae, and single row of short plumose ones flanking ventromesial side of lateral costa; distolateral angle not produced; exopod reaching midlength of carpus.
Rigth chela subovate transversally, not strongly depressed, its width 0.37 times with respect to chela length; surface lacking tubercle and spines, instead bearing punctuations, some of them with small setae, which are arranged in rows in mesial and dorsal surface of palm and mesial surface of opposable finger. Opposable margins of both fingers with band of minute subacute denticles.
Carpus of cheliped owns distal ventrolateral articular condyle with one spine. Width of merus of cheliped 0.37 times on its length, with one conspicuous spine on distal third of ventrolateral side. Ischium without spines or tubercles.
Hooks on ischia of second and third pereiopods, simple and acute, that of third overreaching corresponding basioischial articulation, neither opposed by tubercle on bases. Coxa of fourth pereiopod with prominent caudomesial and cephalomesial bosses; coxa of fifth pereiopod with tuberculiform caudomesial boss.
Sternum between second, third and fourth pereiopods deep; lateral margins not strongly produced ventrally but bearing conspicuous rows of plumose setae.
First pleopods ( Fig. 4 View FIGURE 4 ), as described in “Diagnosis”.
Allotypic female. Differing from holotypic male in the following, other than in secondary sexual features: as usual in members of Cambarellus , chelae more robust, 0.39 times width in its length; abdomen wider, AW 0.22 times in TL; cephalotorax wider, CW 0.21 times in TL; rostral margins more pronounced and strong, forming rostral lateral carina, subrostral ridges also stronger and evident from dorsal view; merus shorter, reaching posterior margin of basal segment of antennal scale. See measurements in Table 1 View TABLE 1 . Annulus ventralis and postannular sclerite ( Fig. 4 View FIGURE 4 ) as described in “Diagnosis”.
Morphotypic male, form II. Differing from the holotype in the following characters: ventrolateral spine on merus less acute, but that on dorsal distal angle more pronounced; hooks on ischia of second and third pereiopods distinctly reduced in size and not reaching baosischial articulation. Terminal elements of first pleopod disposed as in holotype but much shorter and no corneous; mesial process lacking longitudinal sulcus mesialy.
Disposition of types. The holotype (CNCR 28792), allotype (CNCR 28793) and morphotype (CNCR 28794) were deposited at the National Collection of Crustaceans at Instituto de Biología, Universidad Nacional Autónoma de México (CNCR). Paratype series (one male form I, and one female, both under catalog number USNM 1268517) were deposited at the National Museum of Natural History, Smithsonian Institution, USA (NMNH).
Type-locality. Zacapu Lake, in the town of the same name, state of Michoacán, México, 19º 49.336’N, 101º 47.306’ W. The species is only known from this locality. Crayfish were collected by Patricia Ornelas-García and C. Pedraza-Lara on September 2006, along the Southern side of the Lake using dip nets. Specimens were relatively abundant, such that around 30 individuals were obtained in 15 minutes of sampling effort. Individuals were collected close to the margins of the Lake, among the submerged roots of Taxodium sp., submerged vegetation and leaf litter. The lake is formed by a series of springs, so water close to the spring areas is clear.
After a quick consult to the public data bases of three collections (CNCR, NMNH and Museum of Comparative Zoology-Harvard University), two records were obtained of specimens from Zacapu Lake. One of these refer to Cambarellus montezumae (USNM146177) and the other to Cambarellus sp. (IBUNAM:CNCR:CR19032), identification of both records needs confirmation.
Etymology. The name Cambarellus zacapuensis is derived from the specie’s type locality, the Lake Zacapu.
Remarks. C. chapalanus is the most similar species to C. zacapuensis , based on morphological and genetic information as well as personal observations of morphological variation of the rest of species of Cambarellus (Pedraza-Lara et al. 2012) . The new species can be distinguished from C. chapalanus based on the next characters, all of which are more evident when calculated as proportional to other structures of the body (see Table 3 View TABLE 3 ): (all measures in mm) a wider cephalotorax (5.10–5.70 vs. 4.40–4.70), wider abdomen (4.52–4.84 vs. 3.94–4.35) a more robust chela (2.12–2.48 vs. 1.72–1.96) and a shorter merus of cheliped (3.04–4.20 vs. 4.26–4.71), as well as a mesial process of first pleopod of form I male reaching distally well beyond the other elements. Generally, C. zacapuensis possesses a wider body shape and wider body structures than C. chapalanus , as well as show some distinctive apomorphies in genital structure form I male, like a longer mesial process.
Probably related to the significant climatic modifications taken place on freshwater habitats along the TMVB, Zacapu Lake has been proposed as an important site for diversification of several groups of freshwater fauna ( Domiguez-Dominguez et al. 2012; Domínguez-Domínguez et al. 2009; Ornelas-García et al. 2012). This has derived in the recognition of several endemic species, like the fish Allotoca zacapuensis Meyer, Radda & Domínguez, 2002 and Notropis grandis Domínguez-Domínguez, Pérez-Rodríguez, Escalera-Vàzquez & Doadrio , the bivalve Anodonta grandis Say, 1829 and the amphibian Ambystoma andersoni (Krebs & Brandon, 1984) . Vegetation recorded previously for the Lake includes Potamogeton illinoensis Morong , P. pectinatus Linnaeus , Myriophyllum sp., Sagittaria sp., and Ceratophyllum demersum LinnaeusPlants around the Lake include Taxodium sp., Salix sp., Typha latifolia Linnaeus Berula erecta Hudson , Scirpus sp. ( Domínguez-Domínguez et al. 2009). Besides those aforementioned, fish species include ( Domínguez-Domínguez et al. 2009): Alloophorus robustus (Bean, 1892) , Goodea atripinnis , Hubbsina turneri De Buen, 1940 , Skiffia lermae Meek, 1902 , Xenotoca variata (Bean, 1887) , Zoogoneticus quitzeoensis (Bean, 1898), Poeciliopsis infans (Woolman, 1894) , Chirostoma humboltianum (Valenciennes, 1835) , Algansea tincella (Valenciennes, 1844) , and the introduced Cyprinus carpio Güldenstädt, 1773 and Ctenopharyngodon idella (Valenciennes, 1844) .
Genetic distances between C. zacapuensis and its closest relative, C. chapalanus from the basin of Lerma/ Chapala (D ML= 3.6%), would imply a splitting time around 1.3 million years (MY), assuming a cox1 average mutation rate commonly used in crustaceans [1.4% per MY ( Cook et al. 2008; Knowlton & Weigt 1998)]. These dates however, should be taken carefully, as they are assumed from a strict molecular clock of one fragment of evidence only. These dates however, would correspond to a period of separation between the Angulo and Lerma basins during middle Pleistocene, previous to its actual configuration, and is coherent with previous estimation on the formation of the Lake ( Demant et al. 1993). Population studies with genetic markers (Pedraza-Lara et al. 2010) suitable to reveal an accurate degree of population variability would shed more light on the possible zones of contact between C. chapalanus and C. zacapuensis .
Conservation notes. Although under government protection since 2003, multiple disturbances on Zacapu Lake have been documented. Exotic cyprinds inhabiting the lake like common carp may have a negative impact on crayfish populations, as demonstrated for other species in the genus, overall through habitat and behavioural alterations ( Hinojosa-Garro & Zambrano 2004). Demographic drops induced by detriment in habitat quality have been documented in several cases in the Lerma drainage ( Domínguez-Domínguez et al. 2007; Ornelas-García et al. 2012), and could also mean a danger for C. zacapuensis , which is only recorded in one locality. A drastic reduction in water inflow from the spring that feed the Lake has been argued ( Guzmán 1985), indeed, total area of Zacapu Lake was much larger before its premeditated drying for agricultural purposes ( Guzmán 1985). Considering this and that Lake Zacapu is the only known locality for C. zacapuensis , this species is proposed to be considered as critically endangered under the IUCN criteria (CR B-1 a I,iii,iv). Following criteria for national protection in México, C. zacapuensis should be considered as in danger of extinction (14A II, BI, CII, DI).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |