Acanthocasuarina campestris Taylor
|
publication ID |
https://doi.org/ 10.5281/zenodo.278552 |
|
publication LSID |
lsid:zoobank.org:pub:DE18A06F-9AA9-4800-9027-1DC479E72412 |
|
DOI |
https://doi.org/10.5281/zenodo.5620005 |
|
persistent identifier |
https://treatment.plazi.org/id/8D30C212-FF8E-326A-6EA7-C65FFC70C3E7 |
|
treatment provided by |
Plazi |
|
scientific name |
Acanthocasuarina campestris Taylor |
| status |
sp. nov. |
Acanthocasuarina campestris Taylor View in CoL , sp. nov.
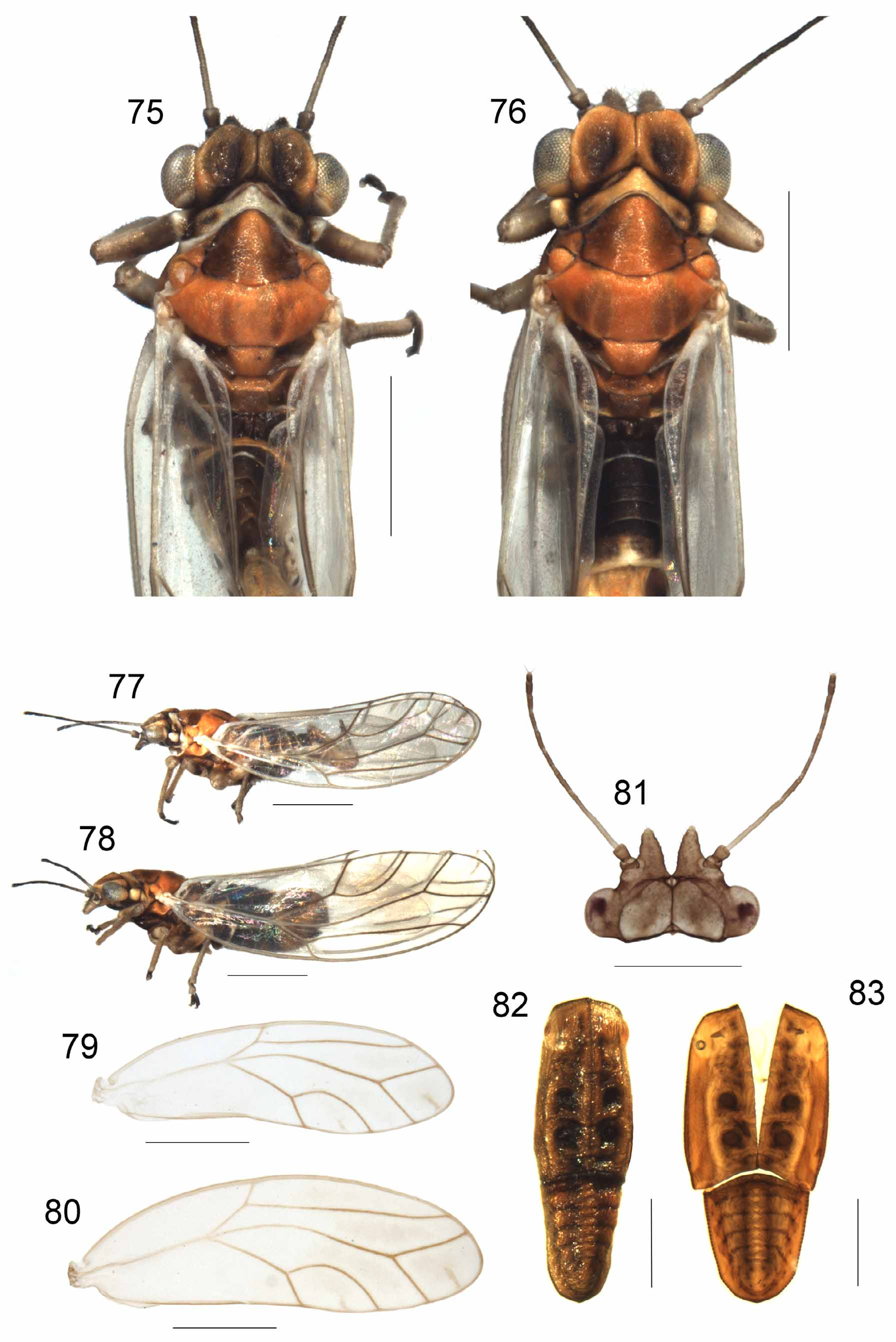
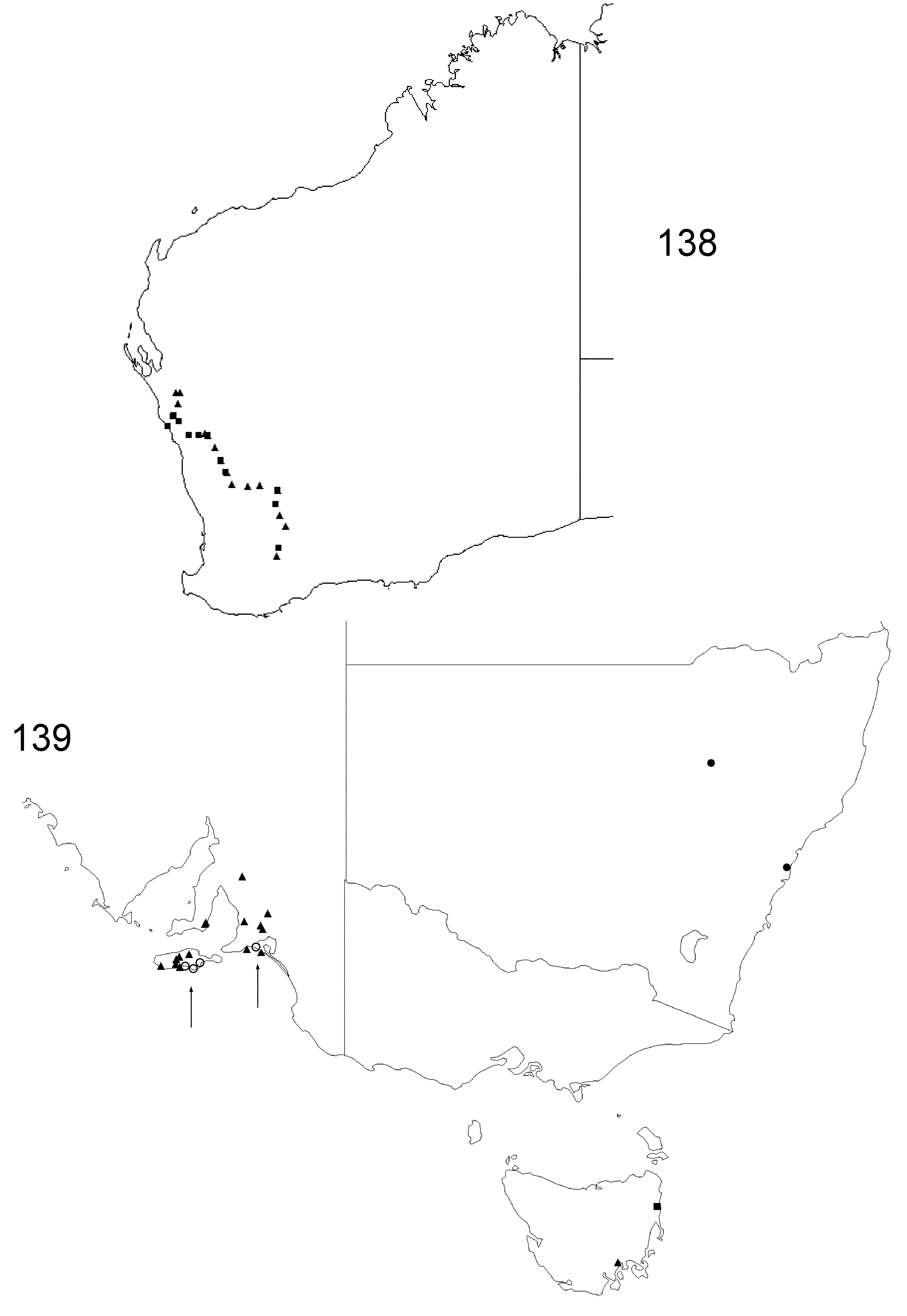
Figs 75–83 View FIGURES 75 – 83 , 97–99 View FIGURES 93 – 102 , 138 View FIGURES 138 – 139 ; Tables 1–8
Types. AUSTRALIA, Western Australia: Holotype: 13 (slide) 15 km S Wialki, 30°37.182’S 118°08.751’E, G.S. Taylor & J.T. Jennings, 6.xii.2008, Allocasuarina campestris , ABCL 2008 656 (WAMA). Paratypes: 8 3, 10 Ƥ (slide), 2 nymphs (1 slide), 2 3, 4 Ƥ (dried), 77 3, 121 Ƥ, same data as holotype (ANIC, WAMA, WINC).
Other material examined. AUSTRALIA, Western Australia: from Allocasuarina campestris : 8 3 25 Ƥ 45 km E Geraldton ( WINC); 11 3, 36 Ƥ Indarra Spring NR ( WINC); 29 3, 31 Ƥ Kalbarri NP ( WINC); 2 3, 1 Ƥ 20 km E Kondinin ( WINC); 1 nymph (dried), 5 3, 12 Ƥ 10 km SSW Mukinbudin ( WINC); 1 3, 5 Ƥ, 1 nymph 13 km N Northampton ( WINC); 1 Ƥ 30 km WNW Northampton ( WINC); 1 3, 1 Ƥ 13 km S Perenjori ( WINC); 28 3, 41 Ƥ Wilroy NR ( WINC); 9 3, 10 Ƥ 35 km N Wubin ( WINC).
Description. Adult ( Figs 75–81 View FIGURES 75 – 83 ). Colour: Male: general colour ochraceous to orange-brown with brown to black markings. Vertex with a pair of diffuse broad brown markings; genal processes dark brown to black; antennal segments 1–2 dark brown to black; segment 3 brown, darker apically; segments 4–10 dark brown to black; pronotum pale with a pair of broad dark brown submedial markings; mesopraescutum with a pair of broad, suffuse, submedial dark brown markings; mesoscutum with two pairs of brown longitudinal submedial stripes, lateral-most thinner and paler; mesoscutellum orange; wings clear; wing vein R+M+Cu pale; legs with dorsal dark brown markings; fore and mid-tarsi dark brown to black, hind basitarsi ochraceous, distal segment of hind tarsi dark brown to black; abdominal tergites brown to dark brown; anterior face of proctiger brown; subgenital plate pale with brown infuscation medially and basally; parameres pale with black apices; proximal segment of aedeagus dark brown to black, distal segment ochraceous. Female: As for male, except generally slightly paler; markings on vertex brown, genal processes ochraceous to brown, pronotum, mesopraescutum and mesoscutum pale brown to brown; proctiger with brown infuscation medio-dorsally and apex dark brown; subgenital plate with brown infuscation and brown apex.
Structure: measurements and ratios as in Tables 1–5. Antennae short, 1.32–1.48 times width of head; genal processes elongate, conical, separated at base and becoming increasingly divergent; anterior margin of vertex rounded from dorsal aspect, delineated from genal processes by prominent ridge; vertex with prominent medial suture; pronotum with prominent anterior, medial node; thorax weakly arched, head distinctly wider than pronotum and mesoscutum; fore wings elongate with broadly rounded apex; Rs long, mostly straight except distally, terminating short of wing apex; vein M distinctly sinuate; vein M1+2 terminating well short of wing apex; cell m1 short, broadly triangular, m1 cell value 0.87–1.14; cell cu1 short triangular, cu1 cell value 1.39–2.19; radular areas thin, elongate in cells m1, m2 and cu1; male terminalia as in Figs 97–98 View FIGURES 93 – 102 ; proctiger without lateral expansions; parameres thin elongate, strongly curved inward towards apex. Female terminalia as in Fig. 99 View FIGURES 93 – 102 ; proctiger short with dorso-posterior margin smoothly rounded from lateral aspect and a barely upturned blunt apical process; subgenital plate broad, triangular from lateral aspect.
Nymph ( Figs 82–83 View FIGURES 75 – 83 ): Measurements and ratios as in Tables 6–7 View TABLE 6 View TABLE 7 . Body light-brown with dark brown markings. Eyes reddish brown; head with submedial brown spots; meso-and metathoracic depressions dark brown to black with irregular dark brown markings anteriorly and posteriorly; caudal plate with dark brown infuscation submedially and delineating margins of abdominal tergites. Body elongate; anterior margin of head weakly pointed medially; dorsum of body with a distinct medial longitudinal ridge; caudal plate with hind margin narrowly rounded.
Etymology. Named after the host plant, Allocasuarina campestris .
Distribution. Recorded from inland Western Australia from Kalbarri and Geraldton and throughout the inland “wheat-belt” region east to Wialki and Mukinbudin, and south to Kondinin in south-western Western Australia ( Fig. 138 View FIGURES 138 – 139 ).
Host plant. Recorded from Allocasuarina campestris (Diels) L.Johnson. Allocasuarina campestris occurs as a dense, erect, 1–3 m shrub on sand-plain and lateritic soils, widespread in the wheat-belt of Western Australia from N of the Murchison River to S of Ravensthorpe and E of Esperance ( Wilson & Johnson 1989).
Comments. See Comments under Ac. acutivalvis for diagnoses.
| WINC |
Waite Insect and Nematode Collection |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |