Clathria (Thalysias) sulfocleistochela, Zea, Sven, Rodríguez, Angélica & Martínez, Ana María, 2014
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3835.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:E3F3FD5C-E526-4A66-911F-0FF5D692AAA8 |
|
DOI |
https://doi.org/10.5281/zenodo.6130517 |
|
persistent identifier |
https://treatment.plazi.org/id/336C1F6A-D749-E73E-4AD6-FE4CFE0BFE01 |
|
treatment provided by |
Plazi |
|
scientific name |
Clathria (Thalysias) sulfocleistochela |
| status |
sp. nov. |
Clathria (Thalysias) sulfocleistochela View in CoL new species
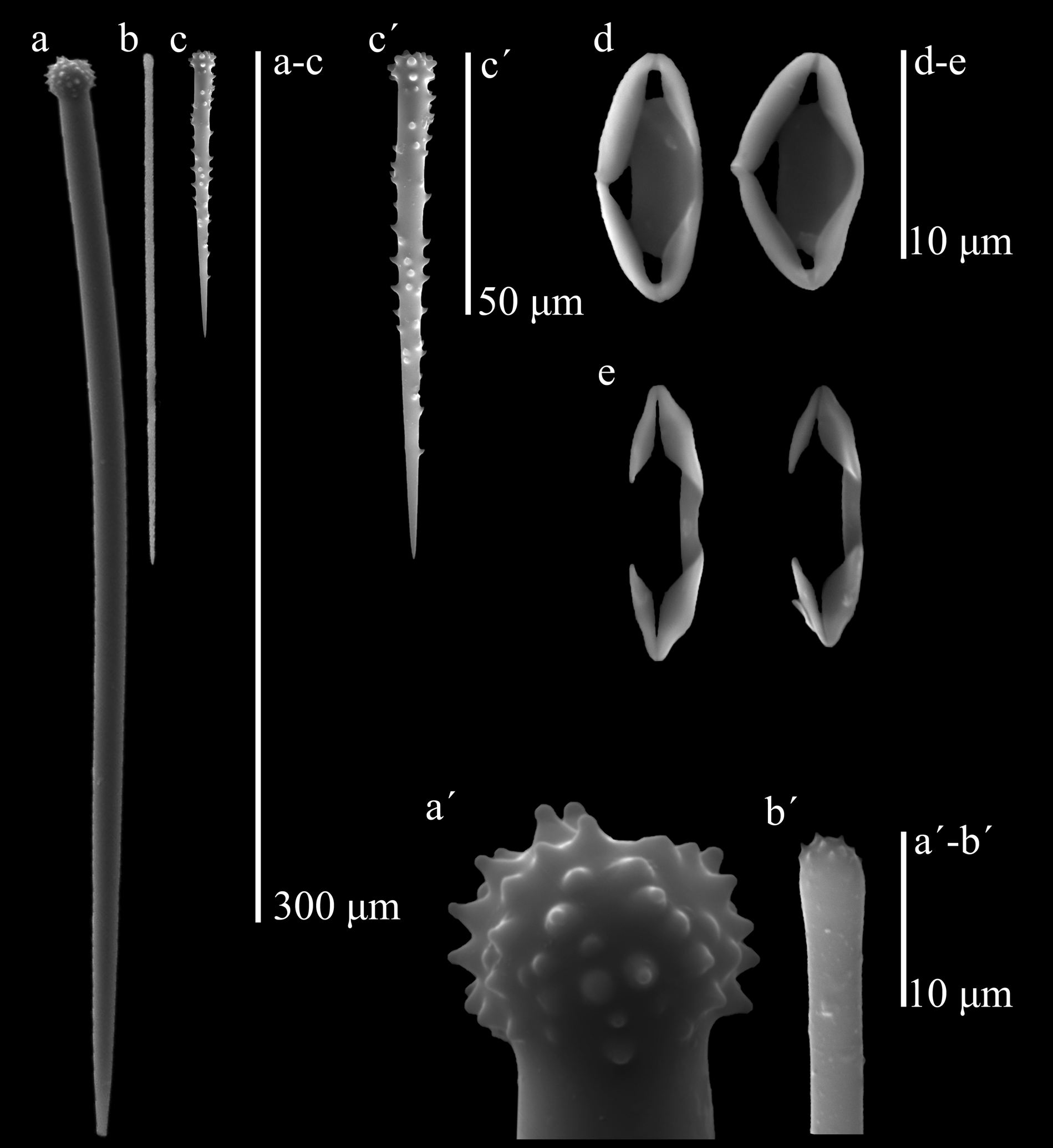
Figures 12 View FIGURE 12 , 13 View FIGURE 13 , plate 2 figures E, F, plate 3 figure D
Clathria View in CoL sp.1; Zea 1993: 83, 88 (ecology).
Rhaphidophlus isodictyoides ; Aerts and van Soest 1997: 129 (ecology). Non: Clathria (Thalysias) isodictyoides (van Soest, 1984) (a valid species).
Material examined. Santa Marta: Holotype: ICN-MHN(Po) 262, Bahía de Santa Marta, Punta de Betín, reef, on crustose coralline algae growing over dead coral, 15 m, coll. S. Zea, 3 Oct. 1983. Paratypes, Bahía de Santa Marta: INV-POR 1248 (4.5–6 m, overhanging rock, rocky shore, 11 Mar. 1988), INV-POR 1249 (28–29 m, dead coral, reef slope, 12 Apr. 1988), INV-POR 1250 (35 m, dead coral, reef base, 19–23 Feb. 1988), Morro, SW side, material of Zea (1993), coll. S. Zea. INV-POR 1251 (18 m, dead coral, reef, 27 Mar. 2012), INV-POR 1252 (12 m, rock, 14 Jun. 2012), Punta de Betín, coll. S. Zea.
Shape, color and consistency. Very thin encrustations extending for several square centimeters over the substratum, often over crustose coralline algae. There is a star-shaped vein pattern of the canal system and oscules, noticeable to the naked eye but relatively small; oscules 1–1.5 mm in diameter, with a low, transparent collar, interspersed and separated 6–12 mm; confluent exhalant canals 380 µm– 1 mm in width. Ectosome transparent; color of subdermal areas between exhalant canals mottled whitish to greenish to sulfur yellow; color beneath canals is dark orange to cinnamon, depending on lightning conditions, apparently owing to the color of the crustose coralline algae that serve as substratum. Consistency soft, difficult to feel due to its thinness.
Skeleton. The ectosome is a thin pinacoderm supported by brushes of smaller auxiliary subtylostyles. In the choanosome, a basal plate of spongin was difficult to distinguish, perhaps because it was embedded in the coralline algal tissue that serves as substratum. At the base there are abundant, erect accessory acanthostyles with their heads touching the first layer of algal cells, and interspersed among them, every 90–200 µm, there are erect principal styles. In thicker areas there are intermediate tracts-brushes of large auxiliary choanosomal subtylostyles; principal styles are also frequently seen horizontally placed among accessory acanthostyles. In very thin areas, the ectosomal brushes of small auxiliary subtylostyles rise directly from the apical half of the styles. Chelae abundantly interspersed in the choanosomal tissue. Spicules (Table 1): (1) Large, slightly curved, thick, basal principal styles with large heads, usually strongly engrossed and spined, sometimes with blunt tubercules; the shafts may have a few spines; ends slightly telescopic, 199– 258.4 –333 µm by 4.7– 8.1 –9.5 µm. (2) Straight choanosomal auxiliary subtylostyles, with heads not very prominent, frequently microspined; there is a wide range of sizes difficult to separate in two categories, although the smaller ones conform most of the ectosomal brushes; large ones, 199– 220.9 –261 µm by 2.9– 3.8 –4.8 µm, smaller ones, 123– 153.9 –185 µm by 1.4– 2.4 –3.8 µm (limit separating the two size categories was set from the larger size observed in the ectosomal brushes). (3) Basal accessory acanthostyles, slender, slightly curved, with a slightly prominent head with many small spines; shaft relatively sparse in spines, apical third usually naked and thin, 59– 109.1 –146 µm by 4.6– 5.2 –6.7 µm. (4) Normal-shaped, palmate chelae, 11.5– 13.5 –16.1 µm. (5) Very abundant, roundish to rhomboidal-shaped palmate cleistochelae (“closed” palmate isochelae, in this case to the point of having the projecting alae fused to each other at the outer tip, and a strongly ridged stem, also fused to the alae), 7.5– 13.2 –16.1 µm, which in some specimens can be slightly larger than normal ones.
Type locality. Punta de Betín, Bay of Santa Marta, Colombia, Caribbean Sea (11° 15’ 2’’ N, 74° 13’ 16” W).
Distribution and ecology. Santa Marta, Colombia. It inhabits rocky shores and coral reefs, 3–36 m in depth, usually only on overhanging substrata at shallow depths (3–8 m), and more exposed and abundant in the deeper reef (14–36 m), where it may cover up to 5.4 % of the hard substrata (cf. Zea 1993).
Etymology. Noun from the latin sulfur for the sulfur yellow tones of its live color, and for the characteristic spicule complement of cleistochelae.
Remarks. This species is indistinguishable in the field from coexisting Clathria (Thalysias) chelosigmoidea n. sp. (described below), although the latter is less common. They are distinguished by skeletal architecture such as usually single erect principal styles in C. (T.) sulfocleistochela n. sp. vs. more commonly in groups of 2–3 arising from a basal spongin sheet in C. (T.) chelosigmoidea n. sp. The main difference in spiculation is the spination in the subtylostyle heads in the former, and the palmate chelae, normal and cleistochelate in the former vs. sigmoid in the latter. We initially hypothesized that they were the same species with spatial or seasonal variation in the cheliferous spicule complement. However, both species were repeatedly collected in the Santa Marta Bay, El Morro, more or less simultaneously in time and at similar depths (4–8 m), first in 1988 during Zea’s (1993) survey, and later in 2012. In general, C. (T.) sulfocleistochela n. sp. reaches deeper waters than C. (T.) chelosigmoidea n. sp.
We examined slides of specimens collected by L. Aerts at Santa Marta (for Aerts & van Soest 1997) and identified by R.W.M. van Soest as Rhaphidophlus isodictyoides van Soest, 1984 (now Clathria (Thalysias) isodictyoides ), and they turned out to belong to C. (T.) sulfocleistochela n. sp. Nevertheless, examination of a tissue slide of the holotype of R. isodictyoides (ZMA POR.4781) revealed that they are different. C. (T.). isodictyoides has palmate chelae with a peculiar central wing in the shaft, different from the rhomboidal cleistochelae of C. (T.) sulfocleistochela n. sp. They differ in color (bright red in the former), and although the other spicules are similar, C. (T.) sulfocleistochela n. sp. lacks the long rhaphidiform toxa present in C. (T.) isodictyoides . Another coexisting species with cleistochelae is Clathria (Microciona) echinata Alcolado, 1984 , which was described by Zea [1987, as C. (M.) simpsoni van Soest, 1984]. It can be distinguished from C. (T.) sulfocleistochela n. sp. in being bright orange, and thinly encrusting, to massive, to tubular. The cleistochela are also slightly different in shape, the alae being flatter and thinner in the former. For an account on the presence of cleistochela in other Clathria species, see van Soest (1984: 99).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Microcionina |
|
Family |
|
|
Genus |
|
|
SubGenus |
Thalysias |
Clathria (Thalysias) sulfocleistochela
| Zea, Sven, Rodríguez, Angélica & Martínez, Ana María 2014 |
Clathria
| Zea 1993: 83 |