Dactylopius gracilipilus, Van, Alex R. & May, Bernie, 2012
|
publication ID |
https://doi.org/ 10.5281/zenodo.283009 |
|
DOI |
https://doi.org/10.5281/zenodo.6169450 |
|
persistent identifier |
https://treatment.plazi.org/id/03F4878D-FFFE-AE51-07BE-FAFB004980A4 |
|
treatment provided by |
Plazi |
|
scientific name |
Dactylopius gracilipilus |
| status |
sp. nov. |
Dactylopius gracilipilus sp. nov.
( Figures 1–4 View FIGURE 1 View FIGURES 2 – 4 )
Material Examined. All specimens are adult females, and deposited in UC Davis, R.M. Bohart Museum of Entomology, Holotype, (1adf) USA, Texas, Brewster Co., Big Bend National Park, N29º08.069’ W103º02.335’, host-plant Corynopuntia schottii , 30-viii-2009, coll. A.R. Van Dam.
Paratypes, (2adff) USA: Texas, Brewster Co., Big Bend National Park, N29º11.570’ W103º01.382’, hostplant C. schottii , 31-viii-2009, coll. A.R. Van Dam. (2adff) USA, Texas, Brewster Co., Big Bend National Park, N29º09.773’ W103º00.554’, host-plant C. schottii , 30-viii-2009, coll. A.R. Van Dam. (2adff) USA, Texas, Brewster Co., Big Bend National Park, N29º08.951’ W103º00.431’, host-pant C. schottii , 30-viii-2009, coll. A.R. Van Dam. (1adf) USA, Texas, Brewster Co., Big Bend National Park, N29º08.069’ W103º02.335’, host-plant C. aggeria (Ralston & Hilsenbeck) Griffith. 30-viii-2009, coll. A.R. Van Dam. (7adff) USA, Texas, Brewster Co., Big Bend National Park, N29º04.499’ W103º06.293’, host-plant C. schottii , 30-viii-2009, coll. A.R. Van Dam.
Description. Live material. Collected from cladodes of Corynopuntia schottii complex ( Benson 1982; Ralston & Hilsenbeck 1992; Griffith 2002) (figs. 2–4). Superficially similar to other Dactylopius (save D. coccus and some populations of D. ceylonicus (Green) , pers. obs.) with typical dense waxy secretions (figs. 2 & 3). Some infestations contained many male puparia.
Adult female.
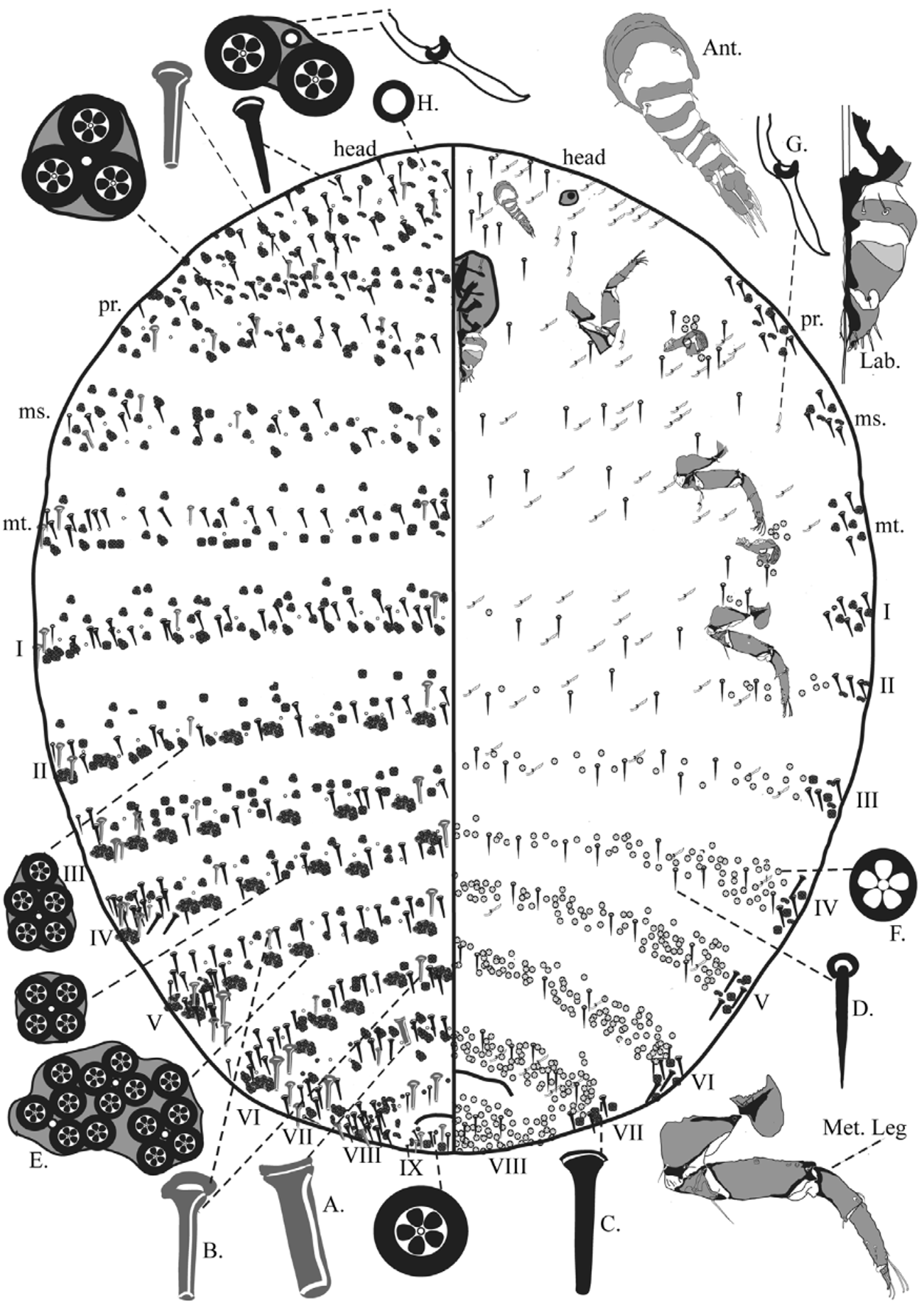
Mounted Material. Body oval, length each 1.95–2.84mm, widest point of metathorax each 1.12–2.38mm. Segmentation distinct on meso- and metathorax, and abdomen. Abdominal segmentation in Fig. 1 View FIGURE 1 numbered as in Williams and Watson (1987).
Antennae six or seven segmented (six segmented in Holotype; segments III and IV fused or partially fused in 10 out of 15 specimens); total length 138–197µm. Segment I each 50–82µm wide, 29–62µm long with three hairlike setae; II 30–47 µm wide, 7–17µm long, with two hair-like setae and one sensorial pit; III 32 –43µm wide, 11– 18µm long, [when fused, IV 22–35 µm long]; IV 30–34 µm wide, 11–16µm long, with two hair-like setae and two sensorial pits; V 21–31 µm wide, 9–15µm long, with one fleshy seta; VI 20–28 µm wide, 10–18µm long, with three hair-like setae and one fleshy seta; VII 19–23 µm wide, 25–34µm long, with four hair-like setae and five fleshy setae.
Tentorial box 219–275µm wide, 214–283µm long, pentagonal, heavily sclerotized. Labium three segmented, 92–169µm wide, 107–155µm long, triangular in shape. Segment I with two pairs of hair-like setae; II with a single pair of hair-like seta on a paddle-shaped projection between segment II and III; apical segment with six pairs of setae, most apical two pairs stout and fleshy, others hair-like.
Spiracles all with a smooth opening, lacking jagged edges, appearing oval, with an inner projection; anterior atrium 47–60µm wide, with 2–7 narrow-rimmed quinquelocular pores and 1 or 2 hair-like setae; posterior spiracle with atrium 43–66µm wide, with 1 or 2 hair-like setae and 2–7 narrow-rimmed pores.
Legs not reduced, typical of other Dactylopius spp., each 310–450µm long, increasing in size posteriorly; each tarsus and claw with two digitules, knobbed at apex, tarsal digitules 37.5–57µm long, ungual digitules 24–41µm long; tarsal claw each with a minute denticle near apex; legs situated on small fleshy lobes, each with 0-2 hair-like setae and 1 or 2 narrow-rimmed quinquelocular pores.
Dorsum. Hair-like setae, each 3.75–7.5µm wide at basal collar, 10–30µm long, smallest on head increasing in size posteriorly towards abdominal segment X; setae scattered anteriorly on head, and prothorax; abdominal segments I–VII, with only 1 or 2 present along margins; abdominal segment VIII with 1 or 2 larger hair-like setae (each approximately 30µm long) near midline and with a single seta submedially; an additional pair present between the margin and submedial setae; abdominal segment IX with a hair-like seta on either side of midline only.
Truncate setae of two distinct types: (i) setae with parallel sides and (ii) setae with sides tapering towards apex. Type (i) truncate setae (shown in gray in Fig.1 View FIGURE 1 ) with parallel sides, each 7.5–10µm wide at basal collar, 10–27.5µm long, smallest on head and increasing in size posteriorly; scattered on head and prothorax, but in two longitudinal lines medially and in indistinct submedial lines from mesothorax to abdominal segment VII (that medially on VII wider than all other type (i) setae, each 12.5–17.5µm wide at basal collar by 20–22.5µm long) plus 1-4 type (i) setae near margins on mesothorax and abdominal segments I-VII; abdominal segment VIII with 1 or 2 type (i) setae on either side of midline, but not submedially; abdominal segment IX with a single type (i) seta nearly adjacent to either side of midline with 2–3 more scattered near margin. Type (ii) setae with sides tapering towards apex, each 5–10µm wide at basal collar, 10–31µm long, smallest on head, increasing in size posteriorly; present in a scattered pattern across head and prothorax, but in distinct transverse bands on meso- and metathorax and all abdominal segments; abdominal segment VII with 1 or 2 near midline; present in clusters of 3-9 on margins of abdomen.
Simple pores each 3µm wide, abundant, scattered across dorsal surface, mainly in indistinct transverse bands on meso- and metathorax and all abdominal segments.
Wide-rimmed quinquelocular pores present mostly in clusters of 2–5 across head and prothorax, but with larger clusters of 5–21 in distinct transverse bands on meso- and metathorax and all abdominal segments except only present singly or in clusters of 2 or 3 in abdominal segment VIII; larger clusters tending to be near margins of abdomen.
Venter. Eyespots each 43–59µm wide, 30–47µm long. Hair-like setae, each 5–7.5µm wide at basal collar 12.5–22.5µm long, smallest on head increasing in size posteriorly; setae tending to be scattered across medial surface of head and thorax but forming indistinct transverse bands across venter of abdominal segments. Narrowrimmed quinquelocular pores present in dense bands on abdominal segments IV–VIII but fewer on abdominal segments II and III and segment I with only 2 narrow rimmed pores close to midline and with a group near margin on II; also present just posterior and anterior to each spiracle. Macrotubular ducts scattered across head through to abdominal segment VII, tending to form transverse bands from mesothorax through abdominal segment VII but with fewer on abdominal segments IV–VI, and absent on abdominal segment VIII.
Etymology. The species name gracilipilus is composed of gracili (Latin) = gracile or slender and pilus (Latin) = hair, the name referring to gracile truncate setae that adorn its dorsum.
Diagnostic features. Dactylopius gracilipilus sp. nov. is most similar in appearance to D. tomentosus , but they are easily distinguish by (character-states on D. tomentosus in brackets): (i) presence of abundant simple pores (absent or rare), (ii) clusters of wide-rimmed quinquelocular pores with10 or more pores abundant (large clusters of wide-rimmed pores uncommon), and (iii) truncate setae with parallel sides forming medial longitudinal rows, each seta 1.5–2 times as long as wide (setae in medial longitudinal row each less than 1.5 times as long as wide).
Remarks. Both D. gracilipilus and D. tomentosus infest members of the tribe Cylindropuntieae sensu Wallace and Dickie (2002) ( Cactaceae ). This might suggest a close affinity between these two species but D. gracilipilus appears to be restricted to Corynopuntia , whereas D. tomentosus has always been found on Cylindropuntia spp., even when Corynopuntia plants are growing about a meter away and are infested with D. gracilipilus . Only a very small portion of desert pavement habitat containing Corynopuntia has been explored for Dactylopius , so there may be other localities in the Chihuahuan, Sonoran, and Mojave Deserts where D. gracilipilus , or even other undiscovered species of Dactylopius , might be found.
Mathenge et al. 2009 showed that, under the conditions of their study, collections of D. tomentosus could be transferred to other Cylindropuntia spp. These authors found that D. tomentosus collections from Cy. fulgida fulgida (Engelm.) F.M. Knuth performed poorly on the host-plant species on which they were not native, and proposed a ‘red queen’ or ‘new association’ type hypothesis for their poor performance (Van Valen 1973; Hokkanen & Pimentel 1984, 1989). Additionally, they hypothesized that Cy. fulgida fulgida plants have no innate immunity to D. tomentosus biotypes originally collected on Cy. imbricata (DC.) F. Knuth and Cy. cholla (F.A.C. Webber) F.M. Knuth , which they used to explain the superior performance of biotypes collected from the latter two host plants on Cy. fulgida fulgida .
There are many alternative host-plant specificity hypotheses ( Janz 2011). Hypotheses incorporating population genetic theory ( Normark & Johnson 2011) suggest that, overtime, isolated populations accumulate mutations on detoxification genes for host-plants that do not occur in their isolated range. This is considered to be a plausible alternative hypothesis for the formation of biotypes, as it would also explain why isolated populations from different biotypes collected in Mexico ( D. tomentosus ‘tunicata’ and ‘rosea’ biotypes) had such poor ability to reproduce on Cy. fulgida fulgida .
Evidence presented by Mathenge et al. 2009 clearly shows that biotypes do exist in Dactylopius even if the evolutionary processes are not clear at present. Additionally, mitochondrial and nuclear DNA gene phylogenies reconstructed by the authors (in prep), including D. tomentosus specimens from much of its known geographic distribution and from similar locations to those used by Mathenge et al. 2009, such as the ‘cholla’ biotype, clearly define D. gracilipilus and D. tomentosus as separate monophyletic groups. Within D. tomentosus , there is geographic structuring largely defined by different desert biomes (in prep). It seems plausible that biotypes represent geographically isolated populations that receive a negligible amount of gene flow from other populations. Allopatry between different populations of the common ancestor of D. tomentosus and D. gracilipilus may have resulted in genetic drift in detoxification genes leading to host specificity and eventual speciation after extended periods of allopatry. Corynopuntia and Cylindropuntia can have different alkaloid compounds ( Meyer et al. 1980), and this difference may act as part of a reinforcement mechanism between D. tomentosus and D. gracilipilus .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |