Dendrapta nasicola, Marcial Irrigoitia & Taglioretti & T. Timi, 2020
|
publication ID |
https://doi.org/ 10.1590/0001-3765202020180933 |
|
DOI |
https://doi.org/10.5281/zenodo.4624028 |
|
persistent identifier |
https://treatment.plazi.org/id/03E9F85A-6575-3034-FC89-307A1BE4FD64 |
|
treatment provided by |
Diego |
|
scientific name |
Dendrapta nasicola |
| status |
sp. nov. |
Dendrapta nasicola n. sp.
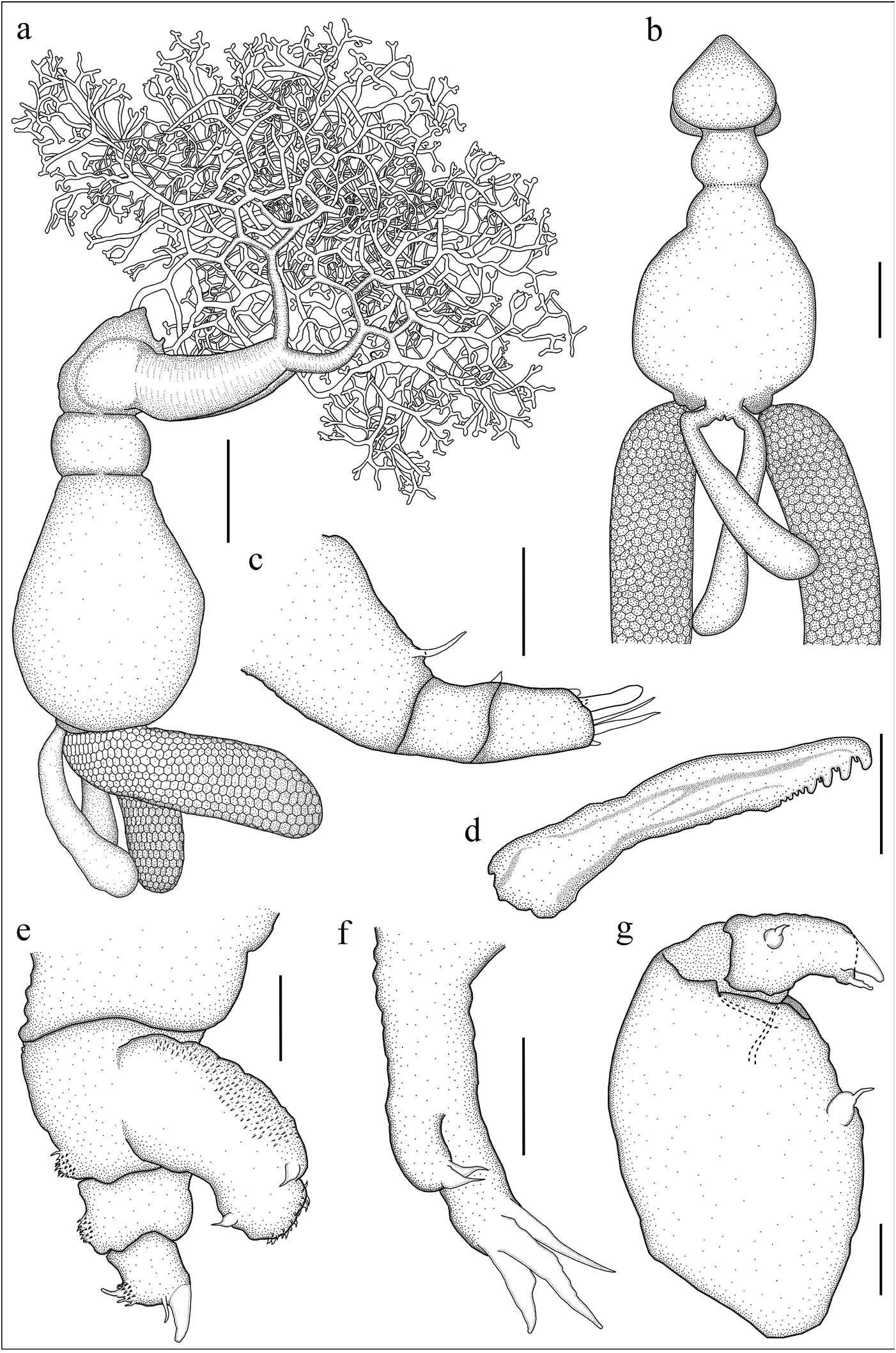
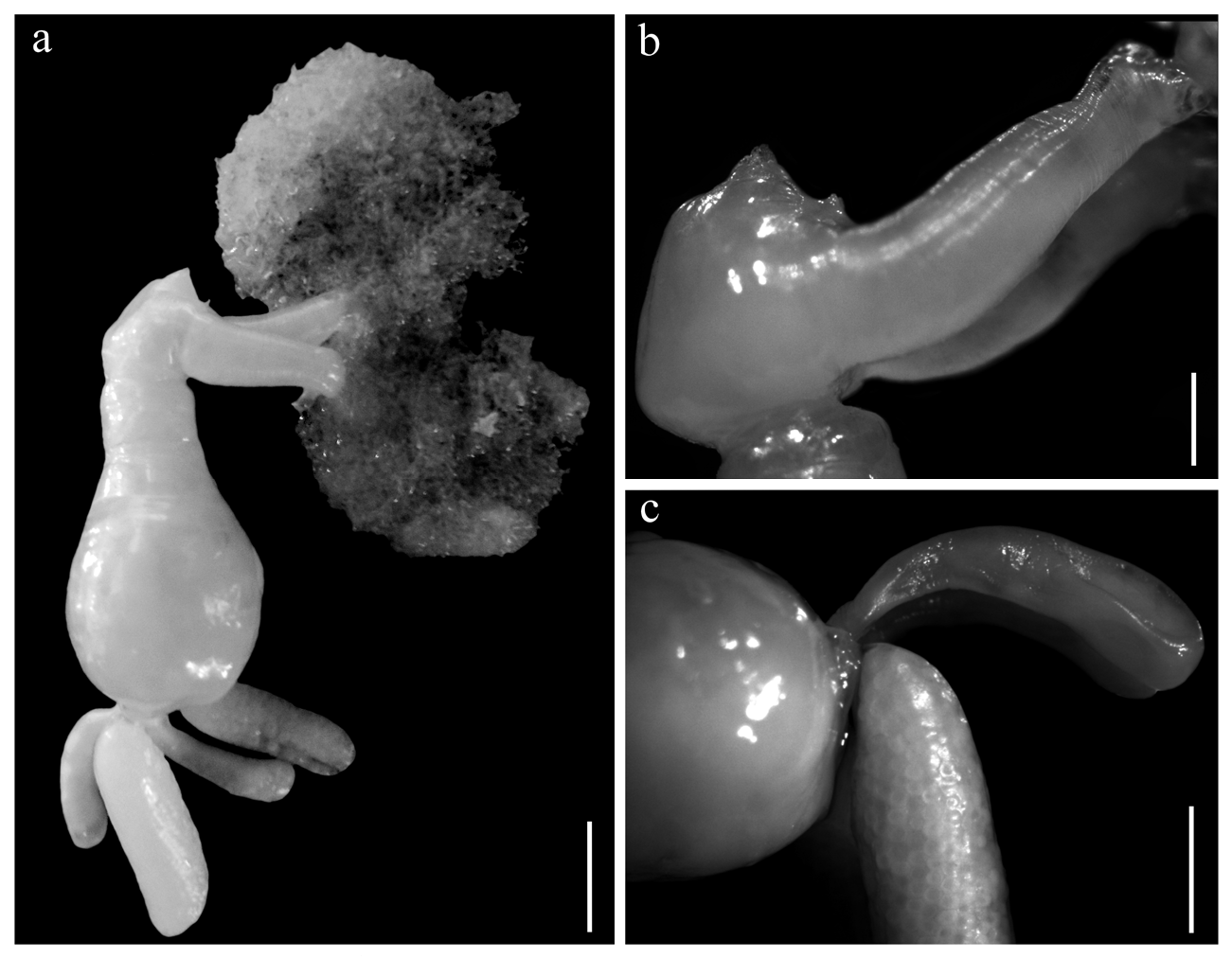
Adult female [based on 15 ovigerous specimens, including holotype, 10 paratypes and four additional specimens used for dissection and SEM]. Total body length (excluding posterior processes) 11.1±1 (9.7–12.8, 15). Cephalothorax heart-shaped, 2.9±0.4 (2.3–3.7, 15) long, 3.2±0.3 (2.9–3.8, 15) maximum width, slightly tilted ventrally tolong axis of trunk ( Figs. 1a, b View Figure ; 2a, b View Figure ), its posterior part fully occupied by maxillary basis ( Figs. 2b View Figure ; 3a View Figure ), dorsal shield indistinct. Buccal cone situated anteriorly on ventral surface of cephalothorax ( Figs. 2b View Figure ; 3b View Figure ). Trunk 8.3±1.1 (6.1– 9.6, 15) long, 5.8±0.5 (5.2–6.8, 15) maximum width, with short anteriorpart (neck), 1.6±0.2 (1.4–2.0, 12) long part separated by shallow constriction from roughly pyriform posterior part, 6.7±0.9 (4.7–7.7, 15) long ( Figs. 1a, b View Figure ; 2a View Figure ). Abdomen small, rather dorsal. Paired posterior processes situated on both sides of abdomen, club-shaped, 6.7±1.0 (5.3–8.9, 27) long, representing 81.0% (62.2–102.4) of trunk length, dorsal to multiseriate egg sacs, 8.5±1.6 (4.9–10.7, 20) long, 2.4±0.3 (1.8–2.8, 20) wide ( Figs. 1a, b View Figure ; 2a, c View Figure ).
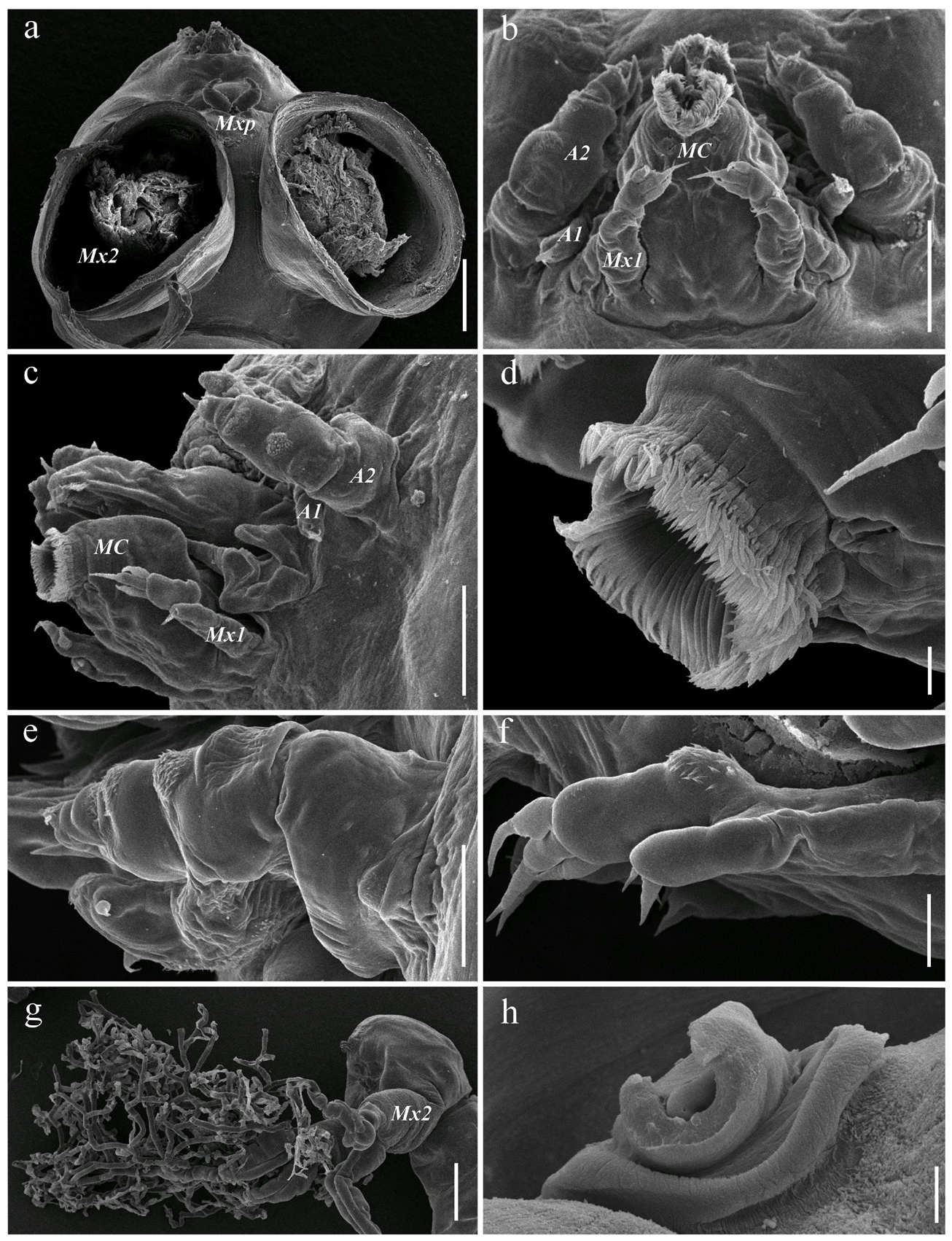
Antennule ( Fig. 1c View Figure ) three-segmented, first segment carrying short dorso-median whip; second segment short, carrying small distal solus, terminal segment with rounded tip, bearing three subterminal tubercles (processes 1-3), digitiform aesthete (4), and two short setae (5 and 6) (armature formula -base to apex- as follows: 1, 1, 5 + 1 aesthete). Antenna ( Figs. 1e View Figure ; 3b, c, e View Figure ) biramous, basis with a distoventral patch of spinules, rami subequally long; exopod 1-segmented with spinulose lateral and distal margins, bearing two short setae (one lateral and one medial) near distal end; endopod two-segmented, first segment with ventral patch of spinules, distal segment armed with robust curved hook 1, slender seta 2, and process 5 located on spinulose process 4 on distoventral margin; process 3 absent. Mouth cone small, labrum and labium fringed by setules ( Figs. 3b, c, d View Figure ); mandible ( Fig. 1d View Figure ) represented by narrow blade with three secondary teeth; dental formula: P1, S1, P1, S1, P1, S1, B5. Maxillule ( Figs. 1f View Figure ; 3f View Figure ) bilobate, exopod cylindrical ending in two subequal setae; endopod longer, with three terminal papillae and proximal patch of spinules. Maxilla ( Figs. 1a View Figure ; 2a View Figure ; 3g View Figure ) developed as a profusely branched holdfast characteristic of the genus, basal part robust ( Figs. 2b, c View Figure ), 4.1±0.9 (2.2–5.4, 26) long, 1.5±0.3 (0.7–2.1, 26), narrowing abruptly before branching in a fragile brush of rhizoid branches holdfast. Vestigial bulla present ( Fig. 3h View Figure ). Maxilliped ( Figs. 1g View Figure , 3a View Figure ) twosegmented. Corpus robust, myxal area with one seta on inflated base. Subchela with basal seta. Claw blunt, slightly curved, barb stout, of similar length than claw.
Adult male not found.
Taxonomic summary
Type-host: Bathyraja scaphiops (Norman, 1937) ( Rajiformes : Arhynchobatidae ).
Type-locality: Deep waters off Buenos Aires province, Argentina (35° - 41°S) .
Site: Olfactory sacs.
Type-specimens: Holotype MLP-Cr coll. No. MLP-Cr 27314 (female). Paratypes MLP-Cr coll. No. MLP-Cr 27315 (10 females) .
Etymology: The specific name refers to the microhabitat of the parasite, the olfactory sacs in nasal cavities of its hosts.
ZooBank registration: This work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the International Commission on Zoological Nomenclature (ICZN). The Life Science Identifier (LSID) for Dendrapta nasicola n. sp. is: urn:lsid:zoobank. org:act:E3E43B22-6237-4CB8-8E3C-30957C951ECB.
Remarks
At present, D. cameroni , the only species in the genus, is represented by two subspecies, D. c. cameroni and D. c. longiclavata ( Dippenaar et al. 2004, Walter & Boxshall 2018). They differ in the proportions of the trunk, in the length of the posterior processes and the mode of branching of the attachment organ ( Kabata & Gusev 1966). Indeed, based on averaged measurements, the trunk of D. c. cameroni is much wider than long, whereas that of D. c. longiclavata is only slightly wider than long; the posterior processes of the former are about half length of the trunk, but more than twice as long as the trunk in the later; additionally, the attachment organ of D. c. longiclavata is much more profuse and more finely divided than in C. c. cameroni ( Kabata & Gusev 1966, Kabata 1988). Despite these differences, normally used as diagnostic specific characters in other species of lernaeopodids ( Boxshall & Halsey 2004), Kabata & Gusev (1966) avoided erecting a new species after observing that, in both forms, females become wider and posterior processes increase in length as individuals grow. Because of the low number of specimens measured at that time, the authors considered that the differences between the two forms could be attributed to developmental variation, not justifying the erection of a different species for the Pacific form; suggesting that their formation must have been caused by recent geographical isolation of their closely related hosts of the genus Raja .
The specimens from B. scaphiops differ from both subspecies of Dendrapta in having a longer, but narrower trunk (trunk length:width= 1:0.7, vs 1:1.4 and 1:1.1 for D. c. cameroni and D. c. longiclavata , respectively). The new species also attains a larger size, but its posterior processes are larger than in D. c. cameroni and notably shorter than in D. c. longiclavata (see also Dippenaar et al. 2004). These differences in morphometric relationships indicates that they are not due to intraspecific variability as a consequence of allometric growth (the larger the specimens, the larger the posterior processes), but they are actual interspecific differences. Indeed, the relative size of posterior processes is considered as a reliable diagnostic character for species of Schistobrachia Kabata, 1964 ( Dippenaar 2016) , a genus closely related to Dendrapta ( Dippenaar et al. 2004) . Additionally, the armature of the antennule of the new species differs from that of its congeners by having a non-bifid tip of aesthete. Base on the observed differences, a new species, Dendrapta nasicola sp. n. is proposed.
Most lernaeopodids are host- and sitespecific ( Piasecki et al. 2010), therefore the host species andthe microhabitat (olfactory bulbs) of the present material, along with the geographic region or origin (Southwestern Atlantic), support the erection of a new species of Dendrapta . For the same reasons, the former subspecies D. c. longiclavata is raised to full specific status and should be correctly known as Dendrapta longiclavata n. comb. Kabata & Gusev, 1966.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Copepoda |
|
Order |
|
|
Family |
|
|
Genus |