Engystomops puyango, Ron, Santiago R., Toral, Eduardo, Rivera, Myrian & Terán-Valdez, Andrea, 2010
|
publication ID |
https://doi.org/ 10.5281/zenodo.197710 |
|
DOI |
https://doi.org/10.5281/zenodo.6200579 |
|
persistent identifier |
https://treatment.plazi.org/id/5D4387F0-C01A-5522-BCE2-FC1CBF59FE44 |
|
treatment provided by |
Plazi |
|
scientific name |
Engystomops puyango |
| status |
sp. nov. |
Engystomops puyango sp. nov.
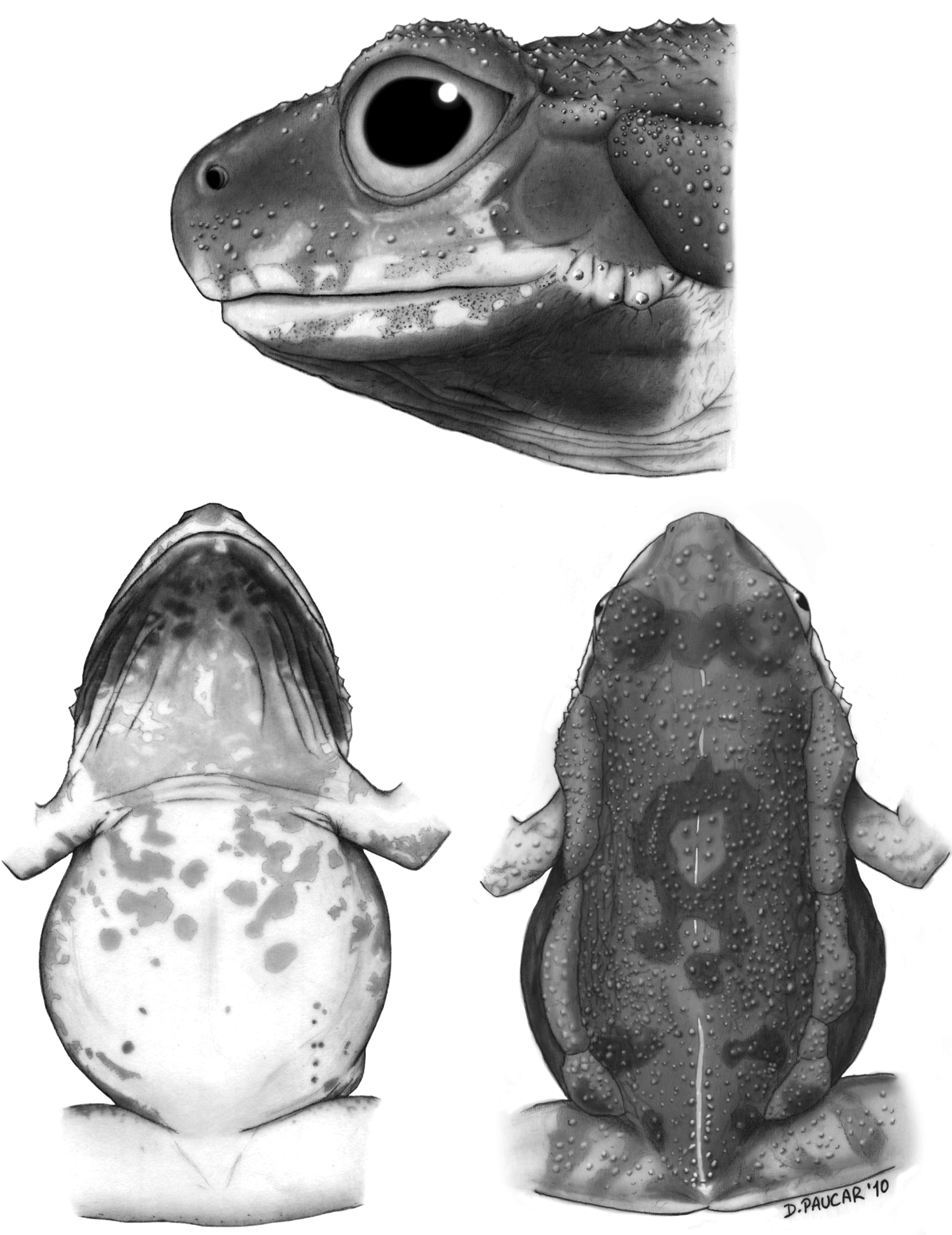
Holotype. ( Fig. 1 View FIGURE 1 ) QCAZ 26978 (field no. PUCE 14586), adult male from Ecuador, Provincia El Oro, Bosque Protector Puyango , near the bridge over the Puyango River on the road Alamor-Arenillas (3.87159° S, 80.03770° W), 321 m above sea level, collected by S. R. Ron, E. E. Tapia, I. G. Tapia on 19 February 2004.
Paratopotypes. QCAZ 26983–84, 26986, 26991, 26996, 26999, 27000–01, 27004, 27006, 27008–14, 27016, adult males, 26987–88, 26990, 27005, adult females, collected by S. R. Ron, I. G. Tapia, and E. E. Tapia on 19 February 2004; QCAZ 26969, 26971–72, 26974–75, 26977–78, adult males, 26967, adult female collected by G. Romero, E. E. Tapia, and S. R. Ron on 7 January 2005; 28717, adult male, 28716, adult female collected by C. Proaño, M. Guerra and S. R. Ron between 13 and 20 February 2005.
Paratypes. Provincia Loja: 10 km north from Zapotillo along the road to Arenillas (4.31198° S, 80.21690° W), 231 m above sea level, QCAZ 26959, adult male, collected by S. R. Ron, I. G. Tapia, E. E. Tapia on 18 February 2004; old road Alamor-Puyango (3.93548° S, 80.10865° W), 650 m above sea level, QCAZ 28758–62, adult males, collected by C. Proaño, M. Guerra and S. R. Ron between 20 and 23 February 2005; Catamayo (3.97004° S, 79.36876° W), 1291 m above sea level, QCAZ 31506–08, adult males, collected by I. G. Tapia and G. Onore on 30 December 2005; Mangaurquillo, FHGO 3372, 3374–76, adult males, collected by F. Nogales.
Diagnosis. A member of Engystomops , clade Duovox. The assignment to Engystomops is based on the molecular phylogeny published by Ron et al. (2006) which shows high support for the inclusion of E. puyango (referred as “ Engystomops sp. D”) within Engystomops . The following morphological synapomorphies ( Cannatella, et al., 1998) also support this assignment: (1) presence of flank glands; (2) presence of parotoid glands; and (3) warty skin.
Engystomops puyango ( Fig. 2 View FIGURE 2 ) is characterized by: (1) mean SVL 27.59 mm in males (range 23.78–30.48; n = 45), 28.68 mm in females (range 25.41–32.68; n = 72); (2) skin on dorsum bearing a mixture of scattered pustules and minute tubercles; (3) snout varying between rounded and protruding in dorsal view and rounded to subacuminate in lateral view; (4) vomerine teeth and odontophores absent; (5) maxillary and premaxillary teeth present; (6) parotoid and flank glands present, usually fused, mean length = 16.63 mm (SD = 1.05; n = 28; 56.9–66.2% of SVL); (7) tarsal tubercle absent; (8) nuptial pads present; (9) Finger I shorter than II; (10) tympanic annulus evident, concealed dorsally and posteriorly; (11) tympanic membrane almost always not tuberculate.
Engystomops puyango Engystomops pustulatus
Chromosome Relative Arm Centromeric Type Relative Centromeric Type number Length ± SD Radio ± SD Index ± SD Length ± SD Index ± SD
Engystomops puyango is most similar to E. pustulatus . They differ in dorsal skin texture (less tuberculate in E. puyango ; Fig. 3 View FIGURE 3 ), chromosomes shape and size ( Table 3 View TABLE 3 ), and advertisement call (shorter and with higher frequency in E. puyango ; Fig. 4 View FIGURE 4 ). Calls are significantly different in total duration (t = 9.68, df = 26, P <0.001), duration of the first component (t = 4.01, df = 24, P <0.001), frequency of the 2nd spectral peak of the call (t = 910.47, df = 43, P <0.001), and frequency of the 2nd spectral peak of the first component (t = 10.65, df = 45, P <0.001; Table 4). Genetically, large distances, typical of species pairs in Engystomops , separate both species. Pairwise uncorrected p -distances between E. pustulatus and E. puyango (range 0.065–0.068) are higher than distances between sister species E. montubio and E. randi (0.027–0.032) or even between nonsister species like E. guayaco and E. montubio (0.059), and E. montubio and E. coloradorum (0.057–0.060). However, genetic distances should not be used as the only evidence for species delimitation ( Vences & Wake, 2007). In our dataset, nevertheless, the genetic data is corroborated by the pattern of differentiation in skin texture, advertisement calls, and chromosome morphology and is consistent with the recognition of E. puyango as a separate species from E. pustulatus .
Engystomops puyango is larger than E. randi , E. montubio , and E. guayaco (non overlapping SVL in adult males) and has less extensive to absent lateral fringes on the toes (fringes are prominent in E. randi , E. montubio , and E guayaco ; Ron, et al., 2004; Ron, et al., 2005). Furthermore, the advertisement call of E. puyango is longer and lacks well-defined pulses at the beginning as in E. randi , E. montubio , and E. guayaco ( Ron, 2008; Table 4, Fig. 5 View FIGURE 5 ). The absence of a tarsal tubercle and the presence of teeth in the maxilla and premaxilla distinguish E. puyango from E. petersi , E. freibergi , and E. pustulosus . Engystomops coloradorum has a shorter advertisement call ( Ryan & Rand, 2001) and more prominent dorsal tubercles (tubercles are smaller and scattered in E. puyango ; Fig. 3 View FIGURE 3 ). Engystomops coloradorum further differs from E. puyango in having a vertical loreal region (oblique in E. puyango ).
Description of holotype. Adult male, 27.33 mm SVL, tibia length 12.43 mm, femur length 11.84 mm, arm length 6.17 mm, head length 8.82 mm, head width 9.02 mm, eye-nostril distance 2.7 mm, head narrower than body except in scapular region; diameter of eye 2.2 times diameter of tympanic annulus; tympanic membrane and tympanic annulus barely evident; tympanic annulus ovoid, longer dorsoventrally; few small tubercles on anterior margin of tympanic annulus, absent from tympanic membrane; supratympanic fold absent; head between the orbits and intercanthal region flat except for scattered tubercles; snout protruding in profile and slightly truncated in dorsal view; nostrils slightly elevated, internarial region convex; canthus rostralis rounded; loreal region concave.
Fingers without expanded discs; nuptial pad present, keratinized, brown, divided in two portions, one covering posterior half of thenar tubercle, other covering Finger I dorsally and posteriorly along its proximal half and posteriorly to distal edge of first phalanx. Base of thenar tubercle ovoid, that of palmar tubercle nearly round; palmar tubercle less prominent than thenar tubercle; subarticular tubercles with round base, all conical; second subarticular tubercle on Finger IV present; few supernumerary palmar tubercles present. Webbing between fingers absent; relative lengths of adpressed fingers III> IV> II> I. Toes without expanded discs; base of inner metatarsal tubercle ovoid, larger than round base of outer metatarsal tubercle; inner metatarsal tubercle more prominent than outer metatarsal tubercle; subarticular tubercles with round base except for proximal tubercles of Toe III and IV (ovoid base), all conical except for subconical distal subarticular tubercles of Toe V; sparse and minute conical and subconical plantar supernumerary tubercles; tarsal tubercle absent. Lateral fringes on toes absent; webbing between toes absent; relative lengths of adpressed toes IV> III> V> II> I.
Skin on dorsum bearing a mixture of pustules and minute, round to subconical tubercles. Some tubercles are arranged in longitudinal rows; skin on venter smooth. Tongue longer than wide; vomerine teeth and odontophores absent. Maxillary and premaxillary teeth present. Vocal slits present, parallel to margins of mandible. Deflated vocal sac forming folds on gular region, extending posteriorly to base of arm.
Color of holotype in preservative. Dorsum grayish brown with lighter gray on the snout; dark gray transversal bar between orbits and irregular dark marks on posterior half of dorsum; light gray middorsal blotch bordered by irregular dark bands at arm insertion axis; dorsal tubercles and pustules light gray; dorsal surfaces of forearms and hindlimbs light gray with dark gray transversal bands. Venter cream yellowish with few minute light gray marks along posterior one-third of the body; Dark-gray blotches on chest and anterior half of abdomen; minute dark gray spots abundant between arms and on posterior half of gular region; anterior half of gular region dark gray with light gray irregular marks close to midline; ventral surfaces of hindlimbs and forelimbs cream to cream yellowish, becoming light gray or cream towards outer and inner edges; some dark blotches present on shanks; outer half of ventral surfaces of forearms dark gray; sides of head gray with ill-defined dark gray marks on the snout and a cream area below the orbit and tympanum, extending posteriorly above deflated vocal folds and anteriorly as a labial stripe; flanks dark gray dorsally, light gray ventrally.
Etymology. The specific name puyango is a noun in apposition, in reference to the type locality, the Puyango Protected Forest. The 2658 ha reserve has one of the world’s largest deposits of petrified tree trunks. It is also one of the largest reserves of tropical dry forest in Ecuador.
Variation. Variation in dorsal coloration of preserved specimens is extensive ( Fig. 6 View FIGURE 6 ). Background dorsal coloration varies from light gray (QCAZ 27005, 27009) to dark gray (QCAZ 31508) or dark brown (QCAZ 35691). Irregular dark marks may be present in diverse patterns ( Fig. 6 View FIGURE 6 ). A clear mid-dorsal line extends along the posterior half of the body (QCAZ 36511) but in some specimens (QCAZ 26988, 26997) it only extends along the posterior one fifth. There is variation in the abundance and arrangement of tubercles (all lighter than the background; Figs. 3 View FIGURE 3 and 6 View FIGURE 6 ). Tubercles coalesce into small ridges that partly enclose a mid-dorsal light blotch in QCAZ 26984, 27005, 27012. The parotoid glands are fused with the flank glands in 28 out of 32 specimens (average gland length is 16.63 mm, SD = 1.05; 56.9–66.2% of SVL). In QCAZ 27013, the glands are fused on the left side but separated on the right side. In QCAZ 26983 and 26989, the flank glands are separated from the parotoid glands on both sides.
Ventral surfaces of preserved specimens have a cream to yellowish-cream background color with light gray (QCAZ 26991, 28762) to dark gray markings (QCAZ 26874, 26984). Marks are arranged in diverse patterns ( Fig. 6 View FIGURE 6 ) and vary from being restricted to the anterior half of the body (darker on folded vocal sacs; QCAZ 26968) to being present over the entire venter (less abundant posteriorly, QCAZ 27008). In a few specimens, the ventral marks are arranged in well-defined large spots (QCAZ 27002, 26983). A midventral cream stripe can be present from near the tip of the snout to the gular region (QCAZ 26994) or continue to the mid venter (QCAZ 26988). In most individuals the stripe is absent (QCAZ 27008) or ill defined (QCAZ 26986). The arrangement of dark spots and tubercles on the ventral surfaces of the feet and hands of QCAZ 26975 is shown in Figure 7 View FIGURE 7 .
Head shape varies between rounded (QCAZ 34511) and subacuminate (QCAZ 28760) in dorsal view; in lateral view it varies between rounded (QCAZ 26972) and protruding (QCAZ 31506, 26974). Lateral head coloration varies between light gray and dark gray. The area below the eye and tympanum is cream in most specimens (e.g., QCAZ 26987–88) but can be restricted to a thin longitudinal light stripe from the jaw articulation to the parotoid gland (QCAZ 26976). Two or three vertical dark bars can be present in the loreal region and below the orbit (e.g., QCAZ 26968, 26973, 26982). The tympanic annulus is concealed dorsally and posteriorly. With few exceptions (e.g., QCAZ 26984, 34511) the tympanic annulus has tubercles; the tympanic membrane has tubercles in only five specimens of the type series (e.g., QCAZ 26989, 28758).
The following morphometric data pertain to adults. In the type series, the largest male has a SVL of 30.48 mm, and the largest female 32.62 mm; mean male SVL = 27.59 mm (n = 45; SD = 1.17), mean female SVL = 27.98 mm (n = 6; SD = 2.38). Amplectant pairs measured during a behavioral study at Bosque Protector Puyango (not collected) had mean male SVL = 26.76 (n = 72; SD = 1.62) and mean female SVL = 28.68 (n = 72; SD = 1.49). Females were significantly larger than males (t = 6.19, df = 100, P <0.001). Snout-vent length was positively correlated between amplectant males and females (ANOVA F = 19.93, df = 71, P <0.001).
Among the type specimens, Catamayo has the highest male SVL (mean = 29.31 mm, SD = 0.51) followed by Puyango (27.50, SD = 0.95) and Mangaurquillo (26.17, SD = 1.75; Table 5 View TABLE 5 ). Differences in SVL are significant between Catamayo and Mangaurquillo (Tukey’s HSD P <0.001), and Catamayo and Puyango (Tukey’s HSD P = 0.020). All other pairwise comparisons were non-significant (P values> 0.071).
Call. Males call while floating on standing water. Acoustic parameters of the advertisement call of E. puyango are shown in Table 4. The call consists of two obligatory and one facultative components with harmonic structure. The first component is characterized by a slight increase in frequency (mean = 48.0 Hz, SD = 67.24, n = 10) with decreasing amplitude modulation. Sidebands (from amplitude modulation) are evident in each harmonic but they merge at the end of the component (as a result, amplitude modulation ends; Fig. 5 View FIGURE 5 ). The power spectrum along all the call shows three spectral peaks ( Fig. 5 View FIGURE 5 C). Dominant frequency of the call was on the second spectral peak in calls from nine out of ten males (mean = 2022.3 Hz, SD = 30.8).
Dominant frequency was 835.5 Hz in calls from the only male on which the dominant frequency was on the first spectral peak. The third spectral peak has high energy only in calls with the third component (see below). The dominant frequency of the first component was on the second spectral peak in calls of nine out of ten males (mean = 2017.6, SD = 40.5). Dominant frequency was 994 Hz in the only male on which the dominant was on the first spectral peak.
The second component follows immediately and is a whine-like note. Each harmonic is a nearly pure tone with descending frequency ( Fig. 5 View FIGURE 5 ). The average decrease in frequency of the first harmonic is 448 Hz (SD = 45.6, n = 10; measured to the end of the call). The average frequency at the beginning of the second component is 937.7 Hz (SD = 41.0, n = 10) for the first harmonic and 1924 Hz (SD = 67.15, n = 10) for the second. The first spectral peak had more energy than the second in calls of 8 males out of 10 males. The number of visible harmonics in the spectrogram varies between 6 and 12.
A facultative third component can be added after the whine and is characterized by a gradual increase in amplitude and a switch in dominant frequency from the first to the fifth or sixth harmonic ( Fig. 5 View FIGURE 5 ). Average fundamental frequency of the third component is 509 Hz (SD = 24.9, n = 10; Table 4); average dominant frequency is 2851 Hz (SD = 135.5; n = 10). Infrequently, the third component exhibits a doubling in the number of harmonics (as shown in Fig. 5 View FIGURE 5 B). The majority of males may be capable of doubling the harmonics because in captivity we recorded repeatedly calls from eight males and all of them produced at least once a call with doubling. This type of call, however, was produced infrequently: out of 12 recordings of third components from seven captive males, only two presented doubling in the number of harmonics; out of ten males recorded at Bosque Protector Puyango , only one did.
Observations at Bosque Protector Puyango suggest that the third component is produced when another individual approaches the calling male. Our experiment to test the influence of the proximity of other individuals in the production of the third component shown a significant positive effect. The average proportion of calls with third component for accompanied males was 0.682 (SD = 0.1943, n = 6) while the proportion for lonely males was 0.085 (SD = 0.140, n = 6). Differences between proportions were significant (Wilcoxon’s z = -2.207; P = 0.027).
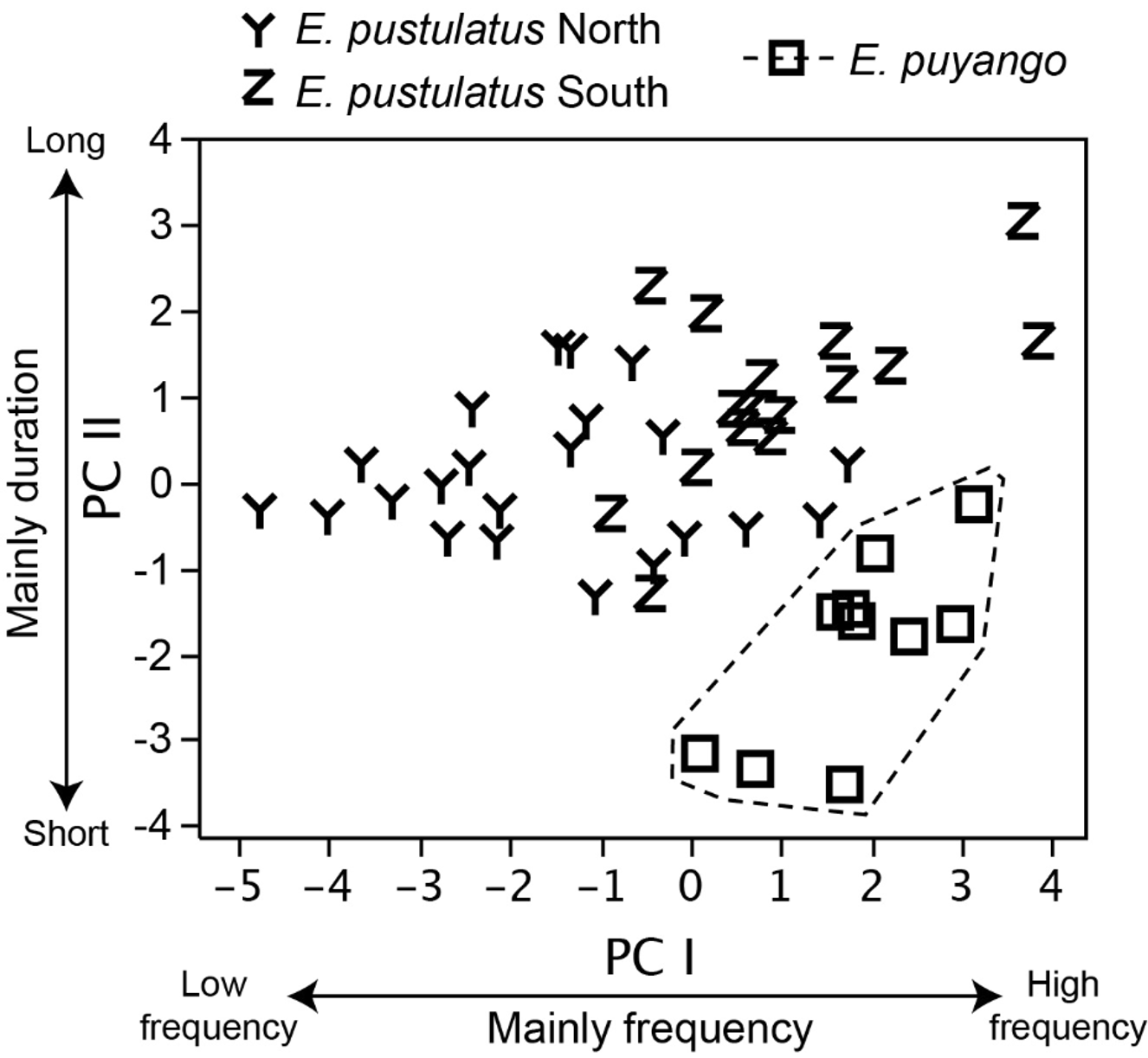
Two components with eigenvalues> 1.0 were extracted from the PCA of calls from 49 males of E. puyango and E. pustulatus . The two PCs accounted for 76.6% of the total variation. Principal Component I loads mostly on frequency variables while PC II on call duration ( Table 2 View TABLE 2 ). The acoustic space of E. puyango is distinctive from that of E. pustulatus as shown by significant differences between both species in scores for PC I (t = 5.34, df = 32, P <0.001) and PC II (t = 6.34, df = 13, P <0.001; Fig. 4 View FIGURE 4 ).
In the DFA classification procedure, calls from all 10 males of E. puyango were correctly classified as E. puyango and calls from all 29 males of E. pustulatus were classified as E. pustulatus . The absence of incorrect assignments confirms the high acoustic distinctiveness between the calls of both species.
Morphometric comparisons. Three components with eigenvalues> 1.0 were extracted from the PCA of 30 specimens of E. puyango , 51 of E. pustulatus , and 11 of E. sp. B. The three PCs accounted for 76.1% of the total variation. The highest loadings were head width and femur length for PC I, arm length for PC II, and head length for PC III ( Table 6 View TABLE 6 ). The morphometric space of E. puyango overlaps with E. pustulatus populations from Arenillas and Huaquillas ( Fig. 8 View FIGURE 8 ) but only slightly with E. pustulatus from Puerto Rico and Patricia Pilar. In contrast, E. sp. B overlaps widely in morphometric space with E. puyango as shown by the lack of significant differences along both PCs (PC I: t = 0.44, df = 23, P = 0.661; PC II: t = 0.55, df = 15, P = 0.587). Comparisons between E. puyango and E. pustulatus of the variables with the highest loadings show significant differences in residual head width (t = 2.20, df = 65, P = 0.03) and residual femur length (t = 2.45, df = 78, P = 0.016) but not in residual arm length (t = 1.38, df = 65, P = 0.17).
In the DFA classification procedure, 16 out of 30 specimens of E. puyango were classified correctly. The misclassified specimens were assigned to E. pustulatus (four specimens) and E. sp. B (10 specimens). Six out of 11 E. sp. B were correctly classified; all misclassified specimens were assigned E. puyango . Overall, the multivariate analyses indicate some morphometric differentiation between E. puyango and E. pustulatus but not between E. puyango and E. sp. B.
Variable Size-free morphology
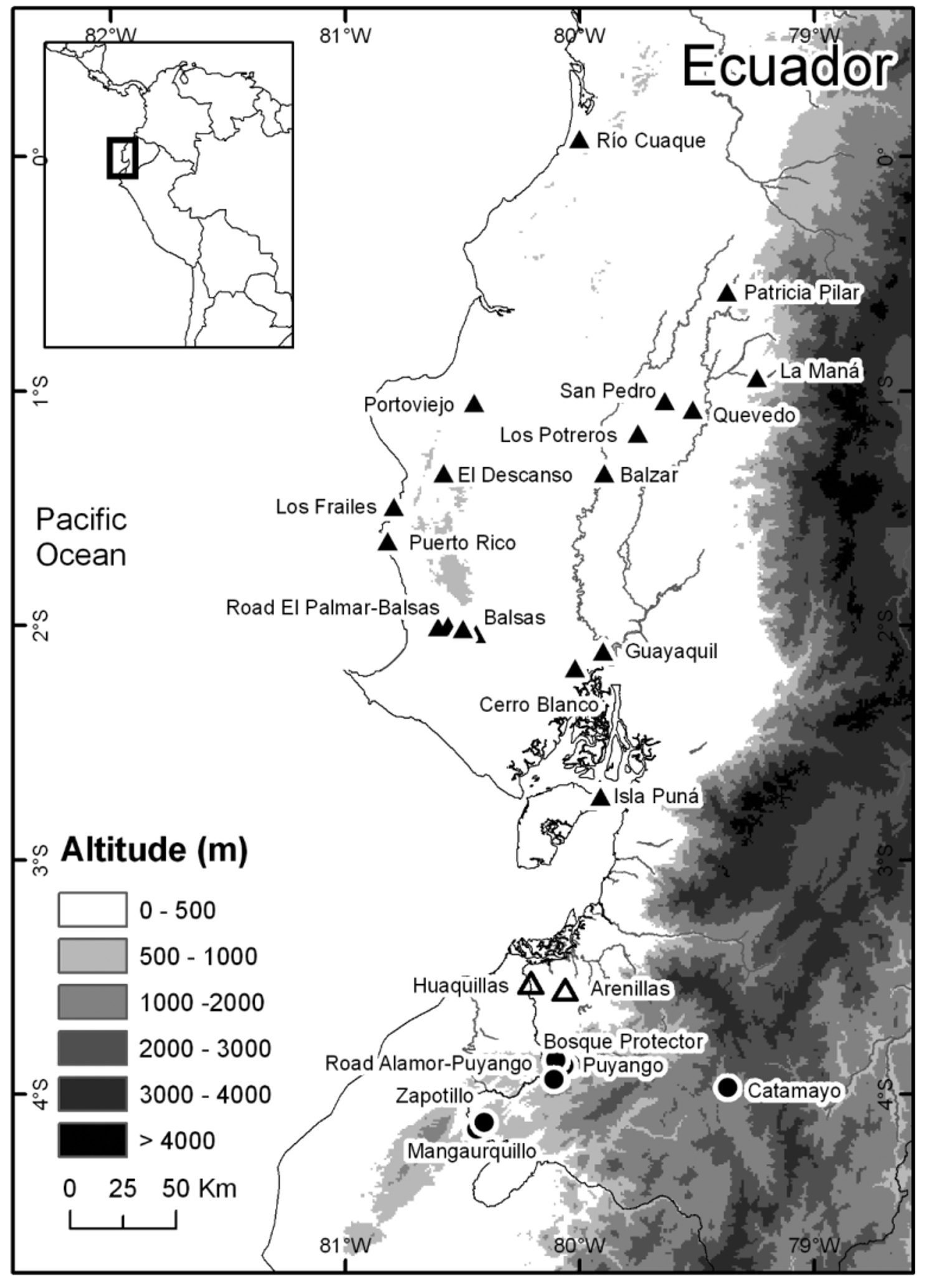
Distribution and ecology. Engystomops puyango has been recorded in southwestern Ecuador (Provincia Loja and Provincia El Oro) between 320–1291 m above sea level ( Fig. 9 View FIGURE 9 ). Maximum straight-line distance between localities is 120 km. The occurrence of E. puyango in Peru is highly probable because Bosque Protector Puyango and Zapotillo are in the border with Peru.
The geographic range of E. puyango is characterized by scant and highly seasonal precipitation. The rainy season lasts between February and April ( Lynch & Duellman, 1997). Localities are in Evergreen Lower Montane Forest of the Western Andes (Catamayo) and Foothill Semideciduous Costa Forest ( Puyango , Mangaurquillo, and Zapotillo; vegetation types are as defined by Sierra, et al., 1999 and Cerón, et al., 1999). The Evergreen Lower Montane Forest is a transitional form between humid forest and the dry forests that predominates in the Andes of southern Ecuador. The Foothill Semideciduous Costa Forest has scattered trees, less than 20 m tall, and a dense understory dominated by herbaceous plants.
All individuals were found in open to sparsely forested areas where the original natural vegetation has been partly or completely removed by humans. At Bosque Protector Puyango , choruses were found in February 2004 and February 2005. Males were calling from water in ponds and ditches. Amplexus and egg deposition take place at the same sites where choruses call. Engystomops puyango constructs floating foam nests during amplexus. While the female discharges the egg masses, the male beats them with his legs to produce the foam.
Males E. puyango and the smaller E. randi were frequently calling at the same ponds, sometimes at distances less than 20 cm. At a dense chorus of both species at Bosque Protector Puyango , on February 19 2005, we found a male (QCAZ 28801) with an unusual advertisement call. Average call duration (0.222 s, SD = 0.006, n = 10) was below the range for E. puyango (Table 4) and close to the average of E. randi (0.206 s, SD = 0.013, based on recordings from 7 males from the same locality). Call interval was closer to that of E. randi (QCAZ 28801 mean = 0.203, SD = 0.011, n = 10 calls; E. randi = 0.324, SD = 0.091, n = 7 males; E. puyango = 1.083, SD = 0.146, n = 10 males). However, similarly to E. puyango , the first component of the call lacked the well-defined pulses present in calls of E. randi ( Ron et al. 2004) and its SVL (28.31 mm) was outside the range of adult males of E. randi (20.09–24.00 mm, n = 24). Because the characteristics of the advertisement call of QCAZ 28801 are intermediate between E. puyango and E. randi , we hypothesize that it is a hybrid individual. A sequence of 750 bases of mtDNA, gene 16S, is identical to that of E. randi QCAZ 23768 from the same population. Thus, the hybrid could be the product of the cross of a female E. randi x male E. puyango . Other species calling in syntopy with E. puyango were Scinax quinquefasciatus View in CoL and Phrynohyas venulosa .
Cytogenetics. All eight analyzed individuals had a chromosome number of 2 n = 20. This is the first known departure from the 2 n = 22 karyotype characteristic of Leiuperidae . All species analyzed of Eladorhina, Eupemphix , Pleurodema , Pseudopaludicola , Physalaemus , and Engystomops have a karyotype 2 n = 22 (e.g., Ananias, et al., 2007; Duellman, 1967; Lourenço, et al., 2000; Lourenço, et al., 2006; Quindere, et al., 2009; Tomatis, et al., 2009). Chromosome pairs 1, 5, 6, 7 and 10 were metacentric while pairs 2, 3, 4 and 8 were submetacentric ( Table 3 View TABLE 3 , Fig. 10 View FIGURE 10 ). Pair 9 was heteromorphic with one chromosome being submetacentric and the other metacentric and slightly smaller. An extended secondary constriction was detected with Giemsa staining in the long arm of two chromosomes 9 ( Fig. 10 View FIGURE 10 A); using the Ag-NOR method, it was determined that the constriction corresponds to the nucleolar organizing region (NOR) ( Fig. 10 View FIGURE 10 B). The difference in size and morphology of pair 9 is a result of the size of NOR: in the submetacentric chromosome 9 the NOR is a large block that doubles the size of the NOR of the metacentric chromosome 9. This heteromorphism in the size of the NOR may be due to varying amounts of its transcription units associated with events of duplication and/or deletion of NOR constituents; NORs are made of highly repetitive ribosomal genes that allow such genetic events. It is also possible that the NOR heteromorphism could result from other mechanisms such as unequal sister chromatid exchange or non-peer linkage in meiosis ( Kasahara, 2009). It could also be the result of differential activity of rDNA transcription, which could explain the existence of inter-cellular polymorphism present in some specimens.
C-banding revealed small blocks of heterochromatin in the centromere of all chromosomes. C-positive bands were observed in the telomeres of the long and short arms of pairs 1 and 5, in the long arm of pairs 3, 6 and 8. Faint intercalated C-bands were observed in the long arms of pairs 1 and 8 ( Fig. 10 View FIGURE 10 C). Pair 9 had C bands in the internal and external borders of the NOR ( Fig. 10 View FIGURE 10 C).
The karyotype of Engystomops puyango shares with E. pustulatus the same number of chromosomes and the position of the NOR in pair 9 (M. Rivera & J. P. Falconí, unpublished). However, there are morphological differences in chromosome pairs 4, 5, and 6 ( Table 3 View TABLE 3 ).
TABLE 3. Morphometry of karyotypes of Engystomops puyango and E. pustulatus. Means and standard deviations are from 25 metaphases from three males (QCAZ 47296, 46932, 47295) and five females (QCAZ 35690 – 91, 46934, 47297, SC 26360) of E. puyango from Bosque Protector Puyango, Ecuador; and four of E. pustulatus (from Cerro Blanco, Ecuador; QCAZ 47031 – 33, QCAZ 46931). Abbreviations are: m = metacentric; sm = submetacentric.
| 1 | 14.08 (± 0.08) 1.36 (± 0.02) | 0.42 (± 0.01) | m | 15.45 (± 0.10) | 0.43 (± 0.03) | m |
|---|---|---|---|---|---|---|
| 2 | 12.80 (± 0.12) 1.81 (± 0.05) | 0.36 (± 0.06) | sm | 12.54 (± 0.09) | 0.37 (± 0.02) | sm |
| 3 | 11.66 (± 0.01) 1.93 (± 0.05) | 0.34 (± 0.04) | sm | 10.9 (± 0.04) | 0.34 (± 0.04) | sm |
| 4 | 10.53 (± 0.17) 2.70 (± 0.06) | 0.27 (± 0.07) | sm | 10.77 (± 0.11) | 0.45 (± 0.05) | m |
| 5 | 9.25 (± 0.22) 1.32 (± 0.04) | 0.43 (± 0.06) | m | 10.44 (± 0.28) | 0.34 (± 0.03) | sm |
| 6 | 9.25 (± 0.09) 1.60 (± 0.03) | 0.38 (± 0.03) | m | 10.03 (± 0.17) | 0.31 (± 0.03) | sm |
| 7 | 9.10 (± 0.07) 1.29 (± 0.04) | 0.44 (± 0.05) | m | 9.02 (± 0.12) | 0.42 (± 0.07) | m |
| 8 | 8.82 (± 0.026) 2.10 (± 0.03) | 0.32 (± 0.02) | sm | 8.62 (± 0.015) | 0.37 (± 0.04) | sm |
| 9a, 9b | 8.61 (± 0.32) 1.63 (± 0.07) 1.77 (± 0.06) | 0.38 (± 0.04) 0.36 (± 0.03) | m sm | 6.52 (± 0.23) | 0.44 (± 0.08) | m |
| 10 | 5.90 (± 0.19) 1.37 (± 0.02) | 0.42 (± 0.01) | m | 5.69 (± 0.22) | 0.43 (± 0.07) | m |
TABLE 5. Descriptive statistics for morphometric measurements of male Engystomops puyango and E. pustulatus used for Principal Component Analysis. Mean ± SD is given with range below. Bold figures are combined for males of all populations. Abbreviations are: SVL = snout-vent length; TL = tibia length; FL = femur length; AL = arm length; HL = head length; HW = head width; EN = eye-nostril distance. All measurements are in mm.
| Species | SVL | TL | FL | AL | HL | HW | EN |
|---|---|---|---|---|---|---|---|
| E. pustulatus (n = 51) | 27.08 ± 1.36 | 11.42 ± 0.59 | 11.50 ± 1.06 | 6.26 ± 0.36 | 8.73 ± 0.42 | 9.11 ± 0.80 | 2.75 ± 0.23 |
| Arenillas (n = 10) | 28.33 ± 0.80 26.84–29.34 | 11.78 ± 0.39 11.24–12.37 | 11.35 ± 0.41 10.85–12.28 | 6.50 ± 0.24 6.22–6.99 | 8.99 ± 0.19 8.76–9.37 | 8.72 ± 0.22 8.34–9.21 | 2.68 ± 0.13 2.37–2.85 |
| Huaquillas (n = 10) | 25.53 ± 1.13 23.90–28.14 | 10.64 ± 0.48 9.98–11.44 | 9.82 ± 0.50 9.02–10.73 | 5.98 ± 0.33 5.25–6.46 | 8.54 ± 0.37 8.08–9.33 | 7.92 ± 0.23 7.56–8.25 | 2.58 ± 0.07 2.49–2.72 |
| Patricia Pilar (n = 17) | 27.43 ± 1.24 25.17–29.88 | 11.59 ± 0.53 10.65–12.72 | 12.09 ± 0.71 10.45–13.01 | 6.24 ± 0.41 5.69–6.84 | 8.73 ± 0.54 7.68–9.6 | 9.60 ± 0.47 8.63–10.28 | 2.84 ± 0.27 2.36–3.42 |
| Puerto Rico (n = 14) | 26.86 ± 0.77 25.85–27.96 | 11.50 ± 0.33 10.94–12.14 | 12.08 ± 0.64 10.44–13.03 | 6.32 ± 0.26 5.76–6.71 | 8.70 ± 0.32 8.15–9.29 | 9.65 ± 0.53 8.76–10.52 | 2.82 ± 0.23 2.31–3.17 |
| E. puyango (n = 30) | 27.31 ± 1.16 | 11.88 ± 0.58 | 11.10 ± 0.64 | 6.36 ± 0.31 | 8.49 ± 0.44 | 8.81 ± 0.61 | 2.65 ± 0.16 |
| Catamayo (n = 3) | 29.31 ± 0.51 28.76–29.79 | 12.31 ± 0.61 11.86–13.01 | 11.84 ± 0.82 11.36–12.80 | 6.80 ± 0.32 6.58–7.17 | 9.11 ± 0.12 9.02–9.26 | 8.50 ± 0.08 8.42–8.59 | 2.65 ± 0.05 2.61–2.71 |
| Mangaurquillo (n = 4) | 26.17 ± 1.75 23.78–27.67 | 11.47 ± 0.19 11.21–11.68 | 10.28 ± 0.45 9.79–10.83 | 6.04 ± 0.23 5.81–6.36 | 8.35 ± 0.52 7.74–8.97 | 9.36 ± 0.37 9.15–9.92 | 2.54 ± 0.21 2.33–2.81 |
| Puyango (n = 23) | 27.43 ± 0.83 25.98–29.29 | 12.03 ± 0.47 11.33–12.77 | 11.29 ± 0.46 10.38–12.19 | 6.42 ± 0.25 5.90–6.84 | 8.50 ± 0.36 7.91–9.45 | 8.80 ± 0.57 7.54–9.8 | 2.68 ± 0.15 2.36–2.92 |
TABLE 6. Character loading and percentage of explained variance for Principal Components (PC) I – II for six morphometric variables. Bold figures indicate highest loadings.
| PC I | PC II | |
|---|---|---|
| Tibia length Femur length Arm length | 0.413 0.526 0.293 | 0.409 –0.128 0.664 |
| Head length Head width Eye-nostril distance | 0.249 0.543 0.328 | 0.073 –0.242 –0.557 |
| Eigenvalue | 2.233 | 1.315 |
| QCAZ |
Museo de Zoologia, Pontificia Universidad Catolica del Ecuador |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Engystomops puyango
| Ron, Santiago R., Toral, Eduardo, Rivera, Myrian & Terán-Valdez, Andrea 2010 |
E. randi (
| Ron et al. 2004 |