Gamelia bennetti Cock and Rougerie, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4942.3.2 |
|
DOI |
https://doi.org/10.5281/zenodo.4619239 |
|
persistent identifier |
https://treatment.plazi.org/id/03F387B1-FFEE-FFAF-FF5C-8A42FC63F8E0 |
|
treatment provided by |
Plazi |
|
scientific name |
Gamelia bennetti Cock and Rougerie |
| status |
sp. nov. |
Gamelia bennetti Cock and Rougerie sp. nov.
urn:lsid:zoobank.org:act:B890CE4B-A62A-4730-8BEC-6A823791DA08
Barcode Index Number (BIN): BOLD:ADW6987; Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 , 6 View FIGURE 6 .
Type material. Holotype ♂: TRINIDAD: TRINIDAD, W.I., Brigand Hill lighthouse, at MV security lights by 22.00h, 17.i.2004, M.J.W. Cock [leg.]/ DNA sampleID MJWC-248, M.J.W. Cock 2018 / Holotype, Gamelia bennetti Cock & Rougerie (to be deposited in NHMUK, ex MJWC).
Paratype, 13. TRINIDAD: TRINIDAD, W.I., Brigand Hill lighthouse, attracted to lights the previous night, 24.iii.2003, M.J.W. Cock [leg.]/ DNA sampleID MJWC-249, M.J.W. Cock 2018 / M.J.W. Cock genitalia 1015 / Paratype, Gamelia bennetti Cock & Rougerie. (to be deposited in NHMUK, ex MJWC).
Both types will be deposited in NHMUK once it is open following the closure for the covid-19 pandemic.
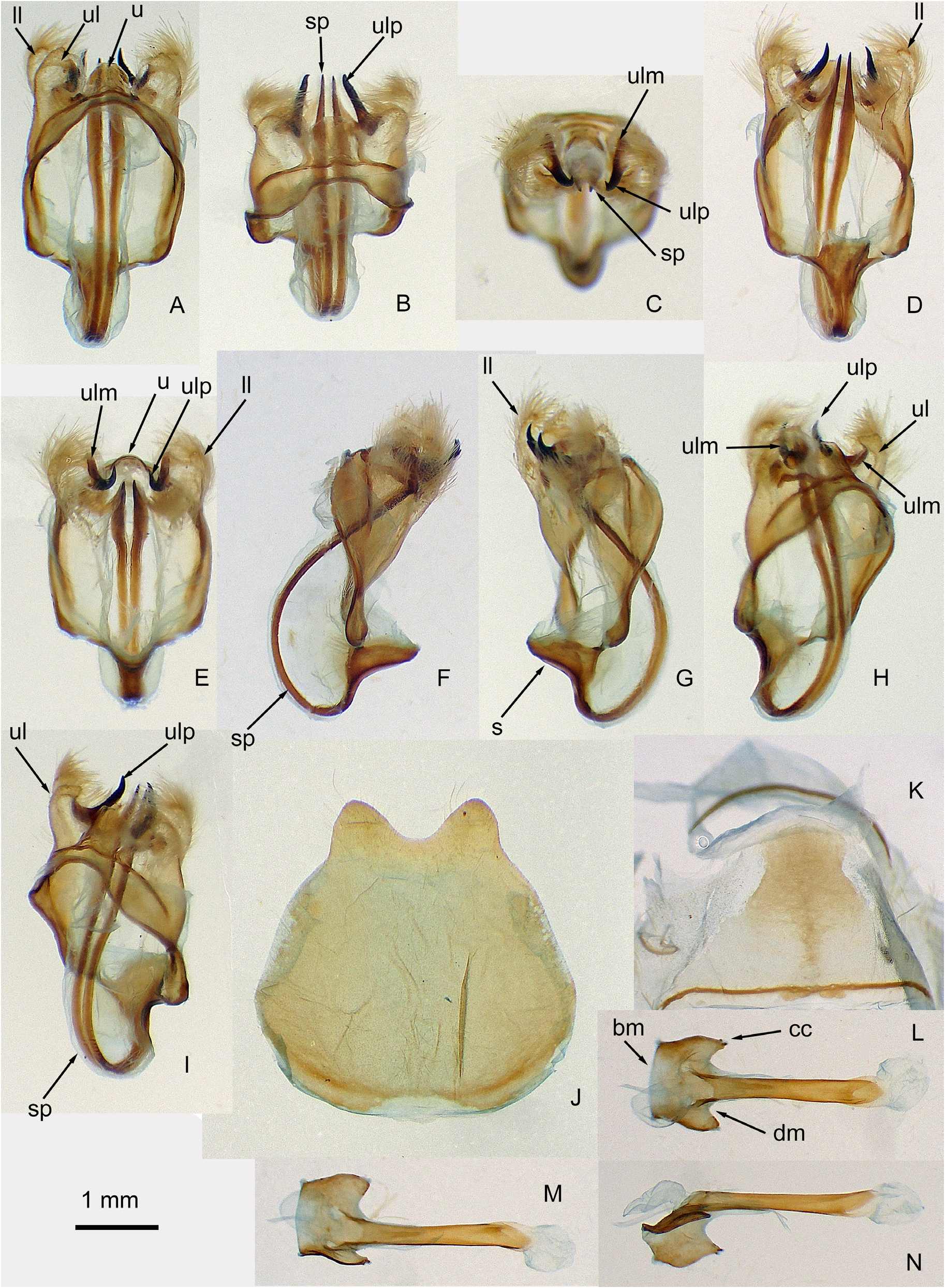
Diagnosis. There are several similar Gamelia species from the Amazon-Guiana-Venezuela area with which this species can be confused, including G. abas , G. rubriluna ( Walker, 1862) , G. lichyi Lemaire, 1973 and G. berliozi Lemaire, 1967 . Given the variability noted between the holotype, paratype and female photo of G. bennetti sp. nov., it is not really possible to point to reliable diagnostic characters of wing markings. The male genitalia are very similar to species in the Gamelia abas group ( Lemaire 2002), particularly G. rubriluna and G. lichyi , and to a lesser extent G. septentrionalis ( Bouvier, 1936) and G. berliozi ( Lemaire 2002, Brechlin & Meister 2012), so we consider G. bennetti sp. nov. to be an additional species of the Gamelia abas group. The genital structure ( Fig. 3 A, D View FIGURE 3 ) is more elongate than that of G. rubriluna , but less so than in the other three species. The saccus ( Fig. 3 View FIGURE 3 D–F) is longer than that of G. rubriluna , but shorter than that of G. lichyi . The long slender lobes of the succus (‘lobes of the vinculum’ in Lemaire (2002)) curl back over the saccus before arching back to emerge under the uncus; it is difficult to compare this curvature with the other species of the group as Lemaire only provides ventral views, and images in Brechlin & Meister (2012) are from microscope slides, whereas lateral or partial lateral views ( Fig. 3 View FIGURE 3 F–I) are needed to observe this character. The saccus lobes of G. rubriluna and G. lichyi joined in their basal half (see figures in Lemaire (2002) and Brechlin & Meister (2012)), but are completely separate throughout in G. bennetti sp. nov. The aedeagus of G. bennetti sp. nov. has a ventral spike ( Fig. 3 N View FIGURE 3 ) as do G. rubriluna and G. lichyi , but not G. septentrionalis and G. berliozi ( Lemaire 2002) . The aedeagus caecum in G. bennetti sp. nov. is a quadrate flange with the distal margin concave ( Fig. 3 View FIGURE 3 L–N), whereas this flange is basally rounded in G. lichyi , G. rubriluna and G. berliozi and the distal margin is concave in G. lichyi , but straight or rounded in G. rubriluna and G. berliozi ( Lemaire 2002; Brechlin & Meister 2012). The sternite of abdominal segment 8 (A8) ( Fig. 3 J View FIGURE 3 ) resembles that of G. rubriluna . The tergite of abdominal segment 7 (A7) ( Fig. 3 K View FIGURE 3 ) resembles that of G. lichyi , and does not have the bottleneck shape of G. rubriluna . At this time, G. bennetti sp. nov. is the only species of the genus Gamelia known from Trinidad, and is only known from the eastern part of the island of Trinidad and perhaps eastern Tobago (see Distribution paragraph). Hence location will give a good pointer as to its identity, although there is no reason to think G. bennetti sp. nov. will not be found to occur more widely in Trinidad or on the mainland in north-eastern Venezuela and/or Guyana. It is therefore fortunate that both the male genitalia and the DNA barcodes can be reliably used to separate G. bennetti sp. nov. from other Gamelia species.
Description. Male. Wingspan of 48–55 mm, and forewing length of 28–30 mm. Head. Dorsal and ventral colour match adjacent forewing ground colour ( Fig. 1 View FIGURE 1 ). Antennae brown (matching dorsal forewing ground colour of paratype), quadri-pectinate, dorsal rami reaching two-thirds the width of ventral rami ( Fig. 1 D View FIGURE 1 ); just over onefifth of forewing length. Thorax. Dorsally matching forewing ground colour; ventrally reddish brown in holotype, orange-brown in paratype. Dorsal forewing dark blackish brown in holotype ( Fig. 2 View FIGURE 2 left) and live photograph ( Fig. 6 View FIGURE 6 ), or brown in paratype ( Fig. 2 View FIGURE 2 right), in all cases darker in basal third which is well covered with dense hair-like setae, especially towards dorsum. An irregular antemedian line and a small dark brown discal spot apparent in paratype, but not in dark brown holotype (although discal spot obvious on ventral forewing). A postmedian line runs from near tornus on anal margin to apex, although hardly visible towards apex; narrow and dark brown with a distal pale margin and then a very narrow dark brown border; much more obvious in brown paratype. Broad marginal band very slightly paler. Dorsal hindwing predominantly grey brown in holotype, but with yellow-brown tone in paratype. A curved, double, postmedian line of dark grey (holotype) or grey (paratype), running from anal margin before tornus to apex; inner line fairly even in width, but outer line broadens considerably in lower half of wing approaching anal margin. Distal to this double line, holotype is uniformly grey-brown, whereas paratype is yellow-grey-brown. Basal to double line, ground colour is paler, with anal area darker and overlaid with hair-like cells. Eyespot displaced inward from postdiscal lines; red with small white centre and broad black border; size variable (compare Figs. 2 View FIGURE 2 and 6 View FIGURE 6 ). Ventral forewing. Holotype dark grey-brown, suffused with russet in basal half, and paler towards anal margin. Antemedian line absent and postmedian line only visible as a slight shadow. Discal spot small, round, and black at the distal end of cell. Paratype similarly marked but ground colour yellow-brown. Ventral hindwing. Ground colour as ventral forewing; eyespot faintly visible through the wing. A straight postmedian line runs from two-thirds on anal margin to external margin just below apex, nearly touching eyespot edge. Abdomen. Dorsally, colour matches marginal band of dorsal hindwing; distally and dorso-laterally it matches basal ground colour of dorsal hindwing; ventrally reddish brown at base, fading to brown distally in holotype; orange-brown basally and yellow brown distally in paratype. Male terminalia (paratype; Fig. 3 View FIGURE 3 ). Central part of posterior margin of A7 tergite flattened; constricted to each side of this before dilating ( Fig. 3 K View FIGURE 3 ). A8 sternite smoothly bilobed on posterior margin ( Fig. 3 J View FIGURE 3 ). Genitalia symmetrical, except as indicated for aedeagus; 3.2 mm from anterior margin of saccus to posterior margin of uncus. Uncus ( Fig. 3 u View FIGURE 3 ) very short (0.35 mm), rounded posteriorly. A long (4.8 mm), thin, pointed process arising from the base of the saccus ( Fig. 3 View FIGURE 3 sp) curves anteriorly, then dorsally and finally posteriorly to finish projecting beyond uncus; processes from each side fused in the basal portion but separate for most of their length. Saccus ( Fig. 3 s View FIGURE 3 ) projects posteriorly, but not anteriorly. Valva bilobed; lower lobe ( Fig. 3 View FIGURE 3 ll) elongate, arching dorsally; upper lobe ( Fig. 3 View FIGURE 3 ul) rounded and partly sclerotised ( Fig. 3 View FIGURE 3 upm) with a strong, curved, sclerotised projection ( Fig. 3 View FIGURE 3 ulp). Aedeagus 2.96 mm long; straight, pointed on dorsal distal margin; vesica simple; caecum of aedeagus a lateral flange each side of base, basal margin ( Fig. 3 View FIGURE 3 bm) straight, lateral margin dilating distally to a point and then concave on distal margin ( Fig. 3 View FIGURE 3 dm); right distal lateral corner with two small teeth ( Fig. 3 View FIGURE 3 cc) ( Fig. 3 View FIGURE 3 L–N).
Provisional description of female. No female specimens were available to us. However, Fig. 4 View FIGURE 4 (left) shows a dorsal view of a living specimen that was not collected, but that is assumed to be the female of G. bennetti sp. nov. as it differs from the male in similar ways to other species of the genus ( Lemaire 2002), although it is not impossible that it represents a second otherwise unknown species from Trinidad. Dorsal head and thorax same colour as basal forewing. Dorsal forewing. Compared to holotype male, wing of female more falcate and paler. Antemedian line strongly marked with a pale inner border, most pronounced on costa. Postmedian line strongly marked, double, black and runs all the way to apex. Discal area pale pinkish brown; no discal spot, although there are 2–3 discal dots, and a diffuse pale patch on costa towards apex. Postmedian area grey brown, with distinct border on external margin, similar in colour to discal area. Dorsal hindwing. Similar to male holotype, but generally paler. Eye spot larger, and distally overlies innermost postmedian line; black border proportionally narrower, and white pupil has a black mark in it. Dorsal abdomen matches thorax at base, but remainder matches basal ground colour of dorsal hindwing.
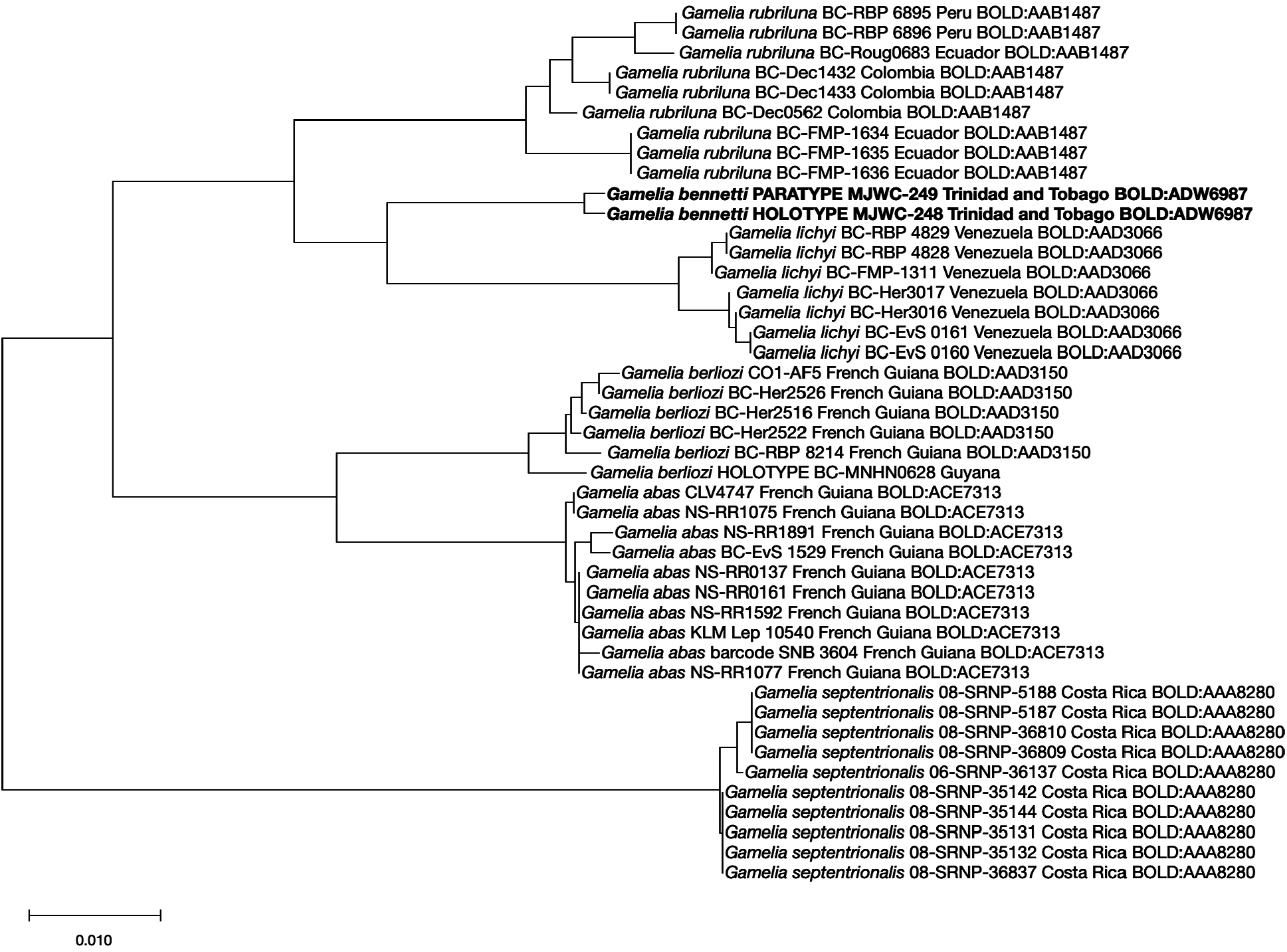
DNA barcodes. The barcodes of the two specimens are almost identical (p-distance of 0.16%); G. lichyi , from Venezuela, is the nearest neighbour to G. bennetti sp. nov., with a minimum p-distance of 3.37% ( Fig. 5 View FIGURE 5 ). Gamelia bennetti sp. nov. is segregated as BIN BOLD:ADW6987.
Variability. Based on the limited observations from Trinidad (two specimens and two photographic records), the male seems to be rather variable with regard to the ground colour, or it occurs in two colour forms, the holotype ( Fig. 2 View FIGURE 2 left) being of a dark blackish brown form and the paratype ( Fig. 2 View FIGURE 2 right) a paler brown form. The dark blackish brown form is seen in the unvouchered images of living males (e.g. Fig. 6 View FIGURE 6 ), and so the specimen of this form was chosen as the holotype. The image of putative females from Trinidad (e.g. Fig. 4 View FIGURE 4 left) indicates a degree of sexual dimorphism in addition. More observations are needed to assess the variation in this species. Lemaire (2002) states that G. lichyi is more variable than G. rubriluna and notes that the lightest males have a bright yellow underside, so it seems likely that G. bennetti sp. nov. will prove to be continuously variable.
An additional photograph of a female from Tobago was located ( Davis 2014, Fig. 4 View FIGURE 4 right). This individual is dark blackish brown, there is a single distinct discal spot, the inner margin of the post median line is pale, and there is a distinct pale subapical patch on the costa. This is likely to be G. bennetti sp. nov., suggesting that the female is also variable, but without a specimen from Tobago to examine, we do not make this assumption. Nevertheless, in almost all cases, the Lepidoptera of Tobago are a subset of the species found in Trinidad, and there are just a few examples of species found in Tobago but not yet in Trinidad, or where Trinidad and Tobago have different subspecies of the same species ( Cock 2017a, 2017b).
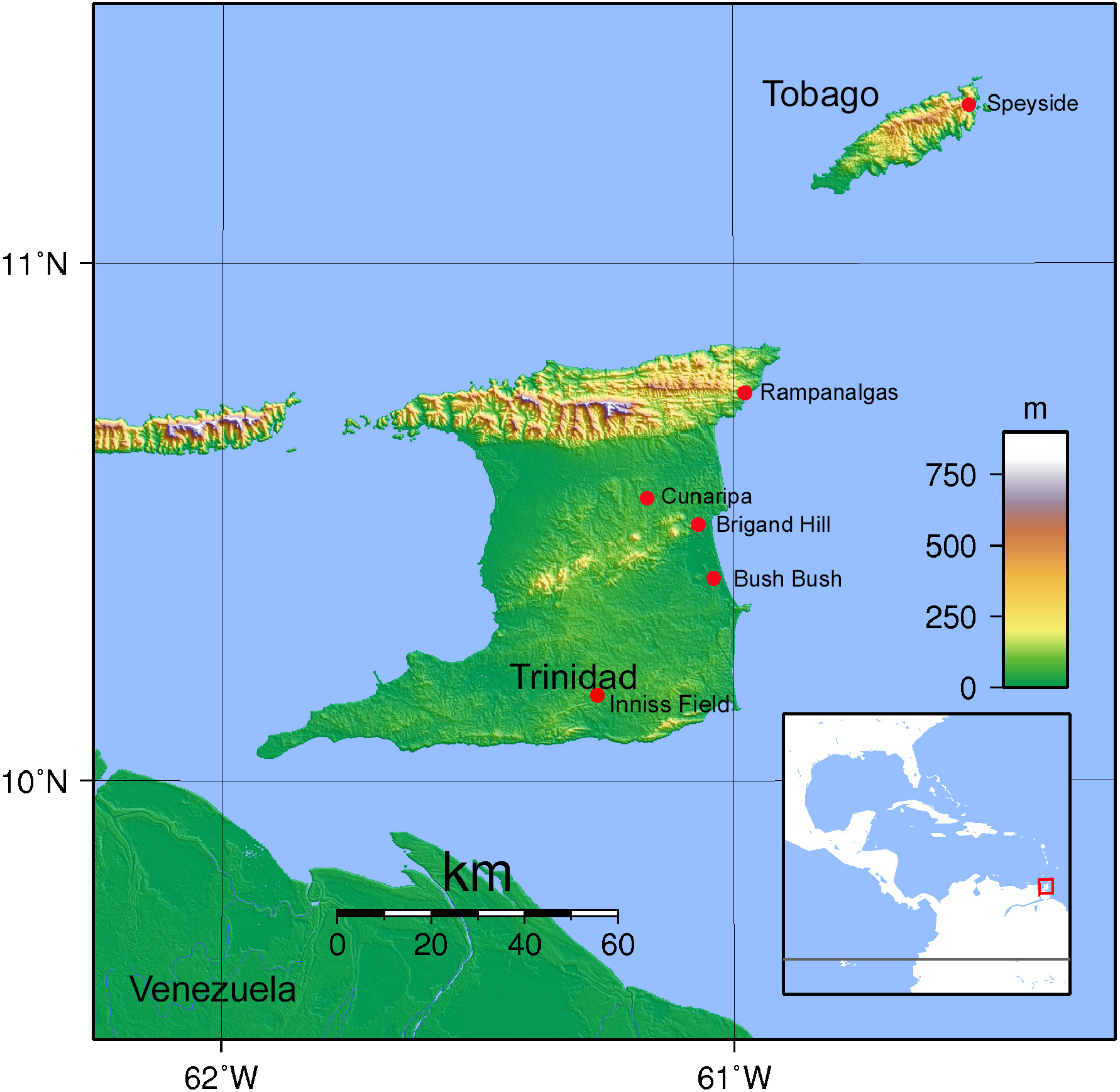
Distribution ( Fig. 7 View FIGURE 7 ). Trinidad and Tobago, Trinidad: W.I., Brigand Hill lighthouse (type series), Bush Bush, Cunaripa, Inniss Field, Rampanalgas (unvouchered photographic records as listed below).
TRINIDAD: Bush Bush : ♂ 18 October 2014 (K. Sookdeo photo) ( Fig. 6 View FIGURE 6 ), ♂ 18 October 2014 (R. Rutherford photo) [iNaturalist observation 38318126] (these two observations are of the same individual); GoogleMaps East of Cunaripa , Bedes Buxoo Trace, by night: ♀ 30 May 2020 (R. Deo photo) [iNaturalist observation 48063102] ( Fig. 4 View FIGURE 4 left); GoogleMaps Inniss Field, 10.17N 61.27W, by night: ♀ 24 December 2020 (R. Deo photo) [iNaturalist observation 67114868] (not shown); GoogleMaps NE of Rampanalgas on Toco Main Road, at light ♂ 26 October 2019 (laurababoolal photo) [iNaturalist observation 34905707] (not shown). GoogleMaps The single photographic record from Tobago ( Davis 2014) probably represents this species, but this needs confirmation: TOBAGO: Near Speyside , + 11.301N, - 60.534W, at light: ♀ 29 November 2014 (P. Davis photo) ( Fig. 4 View FIGURE 4 right) GoogleMaps .
Etymology. This species is named with thanks and appreciation after Dr Fred D. Bennett ( Frank 2019), who was director of the Commonwealth Institute of Biological Control (now integrated within CABI) in Trinidad, during the five years that the first author was based there. Fred’s support, encouragement and help with the study the insects of Trinidad has contributed to the first author’s subsequent four decades long interest in the Lepidoptera of Trinidad and Tobago.
Remarks. This is a rarely seen species in Trinidad, with two collection records and three photographic records, all from the less collected eastern side of the island. The months of capture or observation are January, March, May, October (2) and December in Trinidad, i.e. in both the dry season (January to early May) and the wet season (mid-May to December, often with a short break mid-September to mid-October).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |