Geoplana carbayoi, Amaral, Silvana Vargas Do, Oliveira, Simone Machado De & Leal-Zanchet, Ana Maria, 2012
|
publication ID |
https://doi.org/ 10.5281/zenodo.281355 |
|
DOI |
https://doi.org/10.5281/zenodo.6167356 |
|
persistent identifier |
https://treatment.plazi.org/id/03E4307E-A65F-FFFD-68EA-FCD1FBCDFD6C |
|
treatment provided by |
Plazi |
|
scientific name |
Geoplana carbayoi |
| status |
sp. nov. |
Geoplana carbayoi sp. nov. Oliveira & Leal-Zanchet
Geoplana sp. 3: Baptista, Oliveira & Leal-Zanchet, 2010 Geoplana sp. 3: Baptista & Leal-Zanchet, 2010
Etymology. the specific name honours Dr. Fernando Carbayo Baz and his collaboration in collecting several specimens of land planarians deposited in the scientific collection of the Instituto de Pesquisas de Planárias (UNISI- NOS).
Type material. Holotype: MZUSP PL.01147: I. Fick, leg. 23.VII.1999, Derrubadas (State Park of Turvo), RS, Brazil—anterior tip in three parts: (1) transverse sections on 13 slides, (2) transverse sections on 95 slides, and (3) sagittal sections on 75 slides; anterior region at the level of the ovaries: transverse sections on 81 slides; pre-pharyngeal region: transverse sections on 101 slides; pharynx: sagittal sections on 95 slides; copulatory apparatus: sagittal sections on 96 slides.
Paratypes: MZU PL.00112: M. Cardoso, leg. 20.VII.1999, Derrubadas (State Park of Turvo), RS, Brazil—pre-pharyngeal region: transverse sections on 74 slides; pharynx: sagittal sections on 104 slides; copulatory apparatus: horizontal sections on 74 slides. MZU PL.00113: F. Carbayo leg. 21.VI.1999, Derrubadas (State Park of Turvo), RS, Brazil—pre-pharyngeal region: transverse sections on 35 slides; pharynx: sagittal sections on 67 slides; copulatory apparatus: horizontal sections on 44 slides; MZU PL.00114: F. Carbayo, leg. 21.VI.1999, Derrubadas (State Park of Turvo), RS, Brazil—preserved in ethanol 70o; MZU PL.00115: F. Carbayo, leg. 26.VII.1999, Derrubadas (State Park of Turvo), RS, Brazil—anterior tip: transverse sections on 35 slides; anterior region at the level of the ovaries: transverse sections on 68 slides; copulatory apparatus: sagittal sections on 106 slides.
Type locality. Derrubadas, state of Rio Grande do Sul (RS), Brazil.
Distribution. Rio Grande do Sul (Derrubadas), Brazil.
Diagnosis. Body broad and flat, anterior end pointed, posterior end gradually narrowed. Live specimens with dorsum homogeneously black to the naked eye and venter orange with dark borders. Eyes dorsal, with clear halos; sensory pits in the first third of the body; conspicuous glandular margin; mc:h, 5%–6%; pharynx bell-form; very short esophagus; esophagus: pharynx ratio, 4%–9%; anteriormost testes anterior to ovaries, most posterior ones anterior to pharynx; sperm ducts open into lateral expansions of prostatic vesicle; tubular prostatic vesicle, mainly extrabulbar, consisting of two portions, a laterally expanded T-shaped ental portion, ventrally located, and a sinuous, almost vertically disposed, unpaired ectal portion, with its most distal sinuous part entering the bulbar muscle coat; male atrium relatively short, occupied by dorsal folds of its wall and entally by the truncate, obliquely disposed penis papilla; curved ejaculatory duct opening through the ventral surface of the penis papilla; ovovitelline ducts emerging dorsally and laterally displaced from anterior third of ovaries, and ascending behind gonopore; common glandular ovovitelline duct dorsal to the most ental part of female atrium; vagina as a terminal dorsally directed diverticulum of female atrium; female atrium, oval-elongate, relatively long, and highly folded; length of female atrium, 156% that of male one; gonopore canal posteriorly inclined; no folds separating male and female atria.
Description. External morphology: Body broad and flat, anterior end pointed, posterior end gradually narrowed. When creeping, maximum length may reach 141 mm ( Table 3 View TABLE 3 ). Mouth distance from anterior tip varies from 60% to 65% relatively to body length, gonopore from 72% to 79% ( Table 3 View TABLE 3 ). Live specimens with homogeneously black dorsum. In preserved specimens dorsal colour fades, becoming dark brown. Under the stereomicroscope, dorsal ground-colour light-brown homogeneously covered by dark-brown, dense pigmentation; anterior tip lighter than the rest of the dorsum. Venter orange with dark borders, becoming grayish cream after fixation.
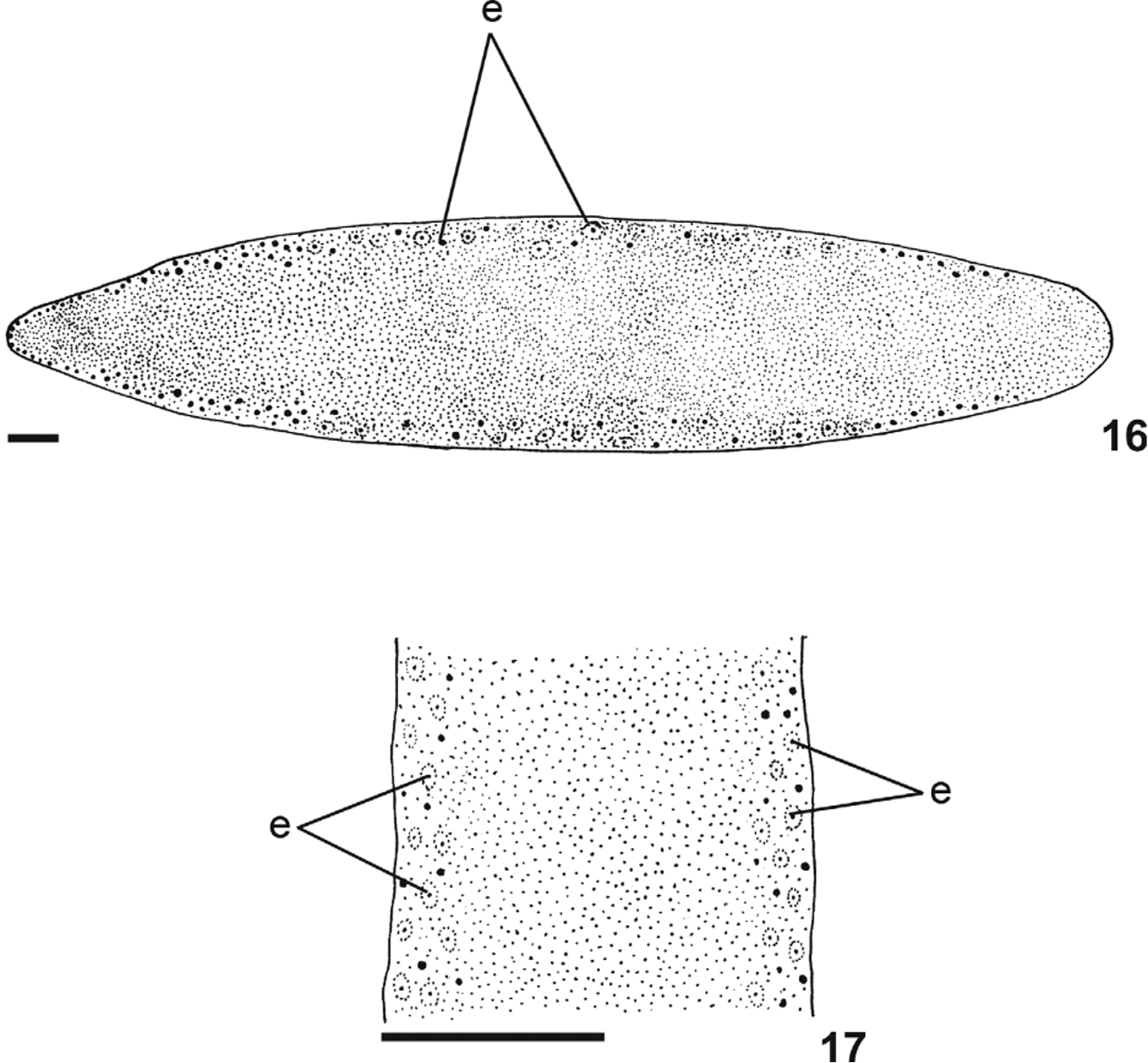
Eyes, initially marginal and uniserial, surround the anterior tip. In paratype MZU PL.00114 they become larger about 1 mm from the tip, forming two series intercalated with smaller eyes, between approximately 2 mm and 5 mm (ca. 2% and 4% of body length, respectively). Between 5 mm and 15 mm (ca. 4% and 12% of body length, respectively), they form two to three series and, after that, the eyes become smaller and appear dorsal with clear halos ( Figs. 16, 17 View FIGURES 16 – 17 ), occupying the maximum width of 2.5 mm in paratype MZU PL.00114 (about 18% of body width on each side of the body). After 55 mm from anterior tip (approximately 46% of body length), they become gradually sparser ( Fig. 16 View FIGURES 16 – 17 ).
Internal morphology. Anterior region: Sensory pits, as simple invaginations, about 35 µm to 48 µm deep, contouring anterior tip, occurring initially at intervals of approximately 25 µm to 35 µm, posteriorly become gradually sparser until they disappear approximately 26 mm from anterior tip (approximately 23% of body length in paratype MZU PL.00115). Eyes (30 µm to 40 µm) initially contour the anterior tip in a single row, and then run along both sides of the body (one or two on each). Cutaneous musculature tripartite, similar to that of pre-pharyngeal region (see next section), but thinner close to the tip; ventral longitudinal fibers absent or very weak in first 0.5 mm of body length. Mesenchymal musculature poorly developed on the anterior tip, with fibers in various directions. Transverse sub-intestinal and supra-intestinal layers appear approximately 1 mm after anterior tip. Rhabditogen cells are rare and sparsely distributed on first 0.5 mm of body length. Three types of secretory cells open through dorsal and ventral epidermis: weakly stained cyanophil cells with amorphous secretion; rhabditogen cells, and erythrophil cells with granular secretion. Only cyanophil cells open through body margins (sensorial border). Small concentrations of erythrophil cells, representing the beginning of the glandular margin, occur ventrally to sensorial border, about 3 mm from the tip (approximately 2.6% of body length in paratype MZU PL.00115).
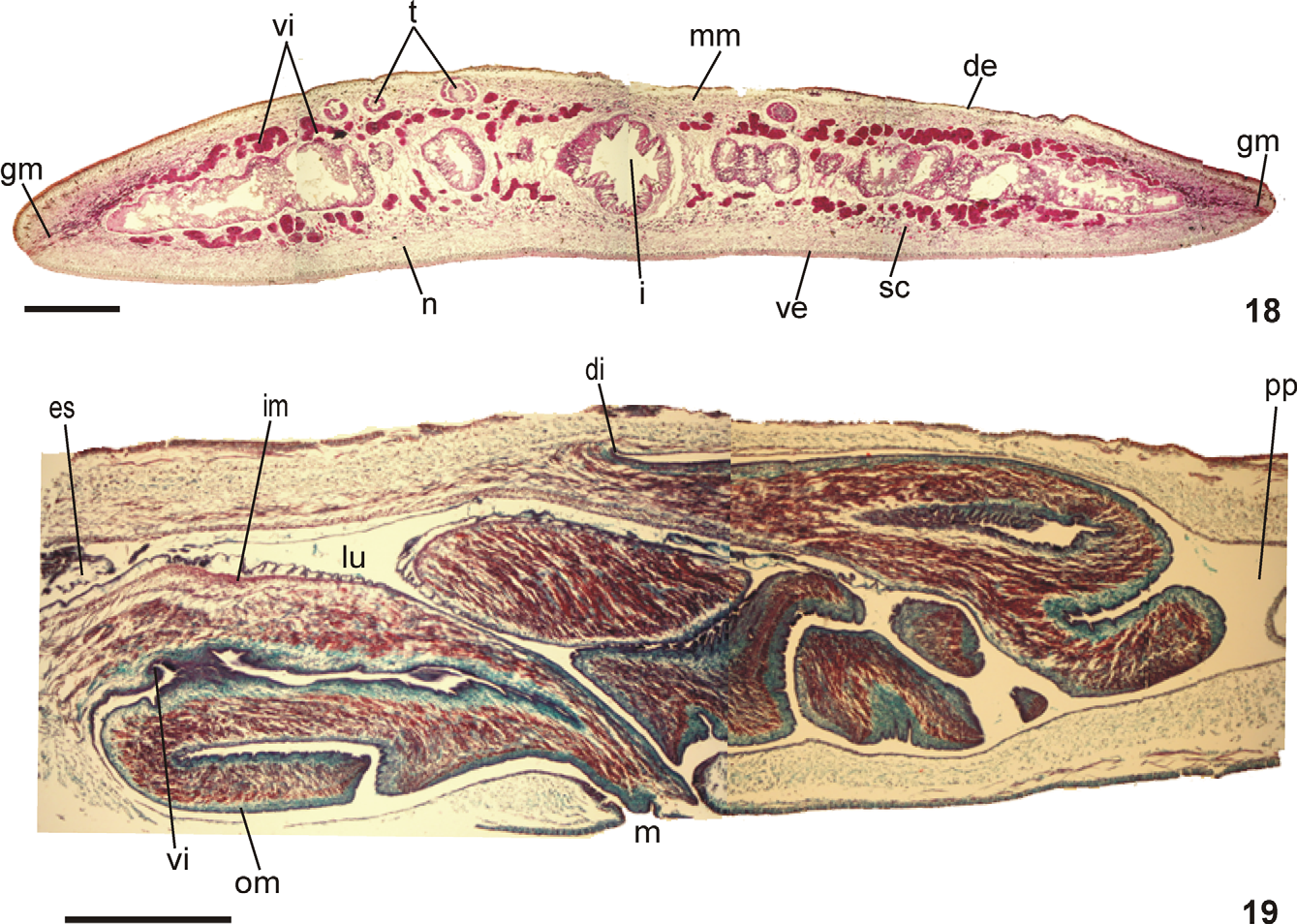
Epidermis and musculature at pre-pharyngeal region ( Fig. 18 View FIGURES 18 – 19 ): Creeping sole broad, 97% of body width, in the holotype. Three types of secretory cells discharge through dorsal epidermis and body margins: (1) abundant rhabditogen cells with xanthophil secretion (2); few cells with fine xanthophil secretion; (3) few cells with cyanophil amorphous secretion. Glandular margin ( Fig. 18 View FIGURES 18 – 19 ) with abundant erythrophil cells with coarse secretion and cyanophil cells amorphous secretion. Creeping sole receives necks of few rhabditogen cells and numerous cells with amorphous cyanophil secretion.
Cutaneous musculature tripartite; longitudinal layer with thick bundles, approximately twice height of circular and diagonal muscle layers. Ventral cutaneous musculature slightly thicker than dorsal musculature at the sagittal plane and paramedianly, but progressively lower towards body margins. Mc:h 5% to 6% ( Table 4 View TABLE 4 ).
Mesenchymal musculature poorly developed composed of three layers: dorsal subcutaneous with oblique fibers variously oriented (ca. 2–3 fibers thick); supra-intestinal transversal (about 3 fibers thick); and sub-intestinal transversal (ca. 4 fibers thick). In addition, scattered subneural transversal and ventral subcutaneous oblique fibers, as well as dorsoventral ones are present. If existent, longitudinal fibers are indiscernible, few and very scattered.
Pharynx ( Fig. 19 View FIGURES 18 – 19 ): Pharynx bell-form with folded margins. Mouth at the level of dorsal insertion in median third of pharyngeal pouch. Esophagus, very short, lined with ciliated columnar epithelium with a few insunk nuclei, receiving some openings of erythrophil secretory cells with granular secretion and bodies located in mesenchyme, with muscularis comprising a mainly circular layer and some interposed longitudinal fibers. Esophagus: pharynx ratio, 4%–9%. Pharynx and pharyngeal lumen lined with ciliated columnar epithelium showing several insunk nuclei. Pharyngeal glands with cell bodies located in mesenchyme, mainly anterior and posteriorly as well as lateral to pharyngeal pouch. Three secretory cell types present: cells with coarse granular xanthophil secretion, cells with fine granular erythrophil secretion, and cells with cyanophil amorphous secretion. Outer musculature of pharynx (ca. 40 µm thick) comprises a thin longitudinal subepithelial layer, followed by a thicker circular one, mixed internally with few longitudinal fibers. Towards pharyngeal tip, circular layer becomes as thin as longitudinal one. Inner pharyngeal musculature comprises a thick circular subepithelial layer mixed mainly externally with some longitudinal fibers (135 µm thick). Inner musculature gradually tapers outwards and dorsomedially.
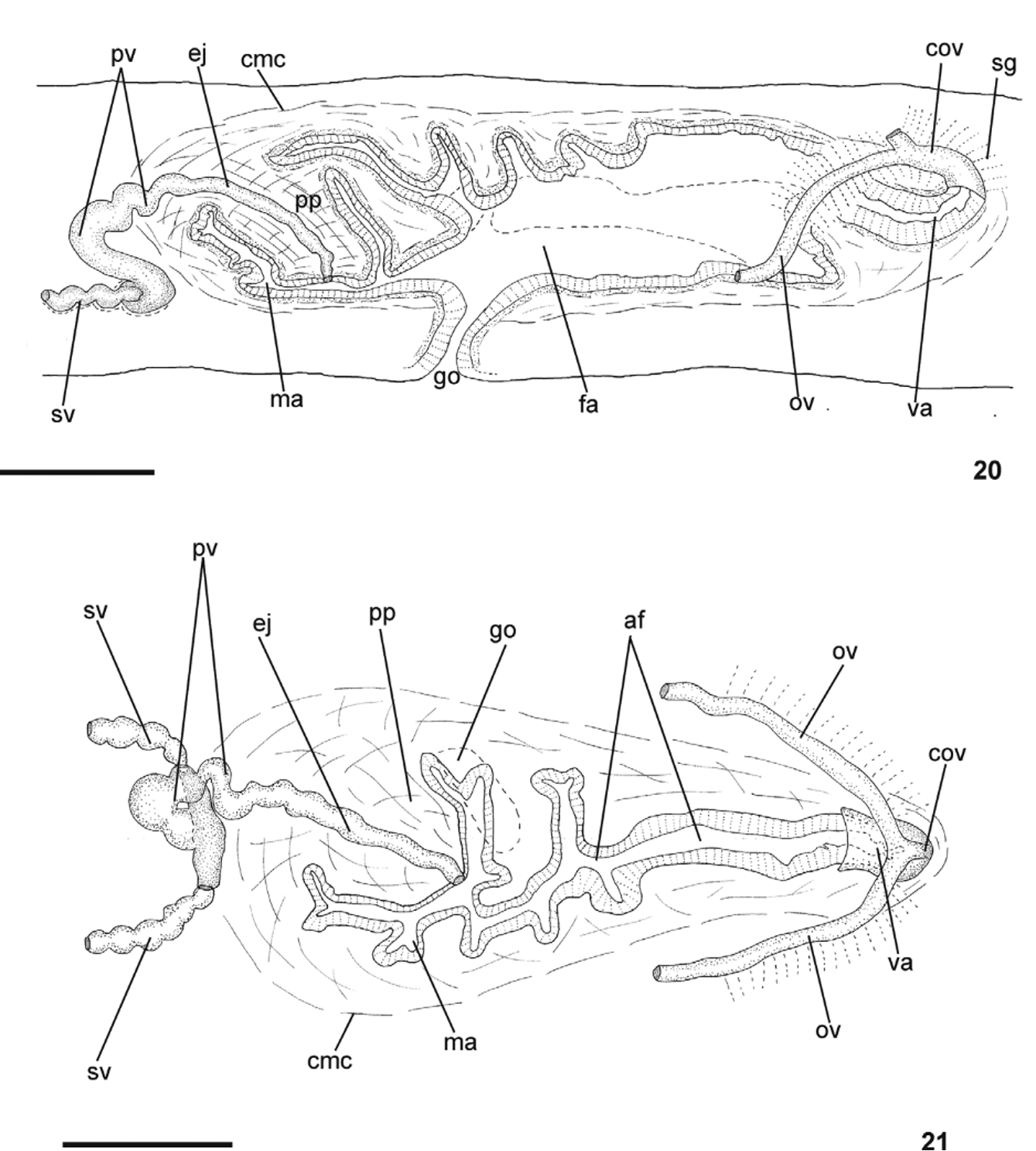
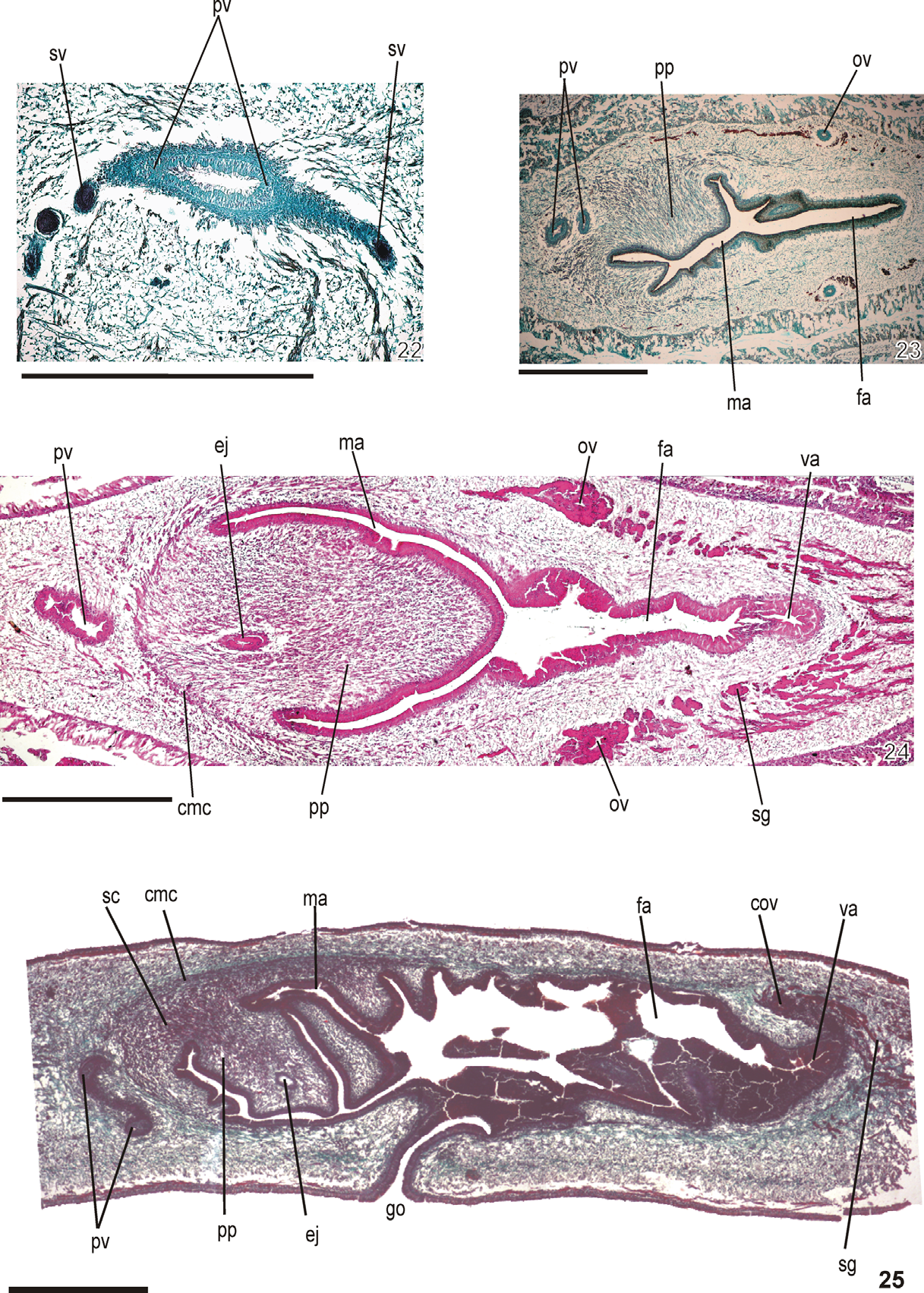
Reproductive apparatus: Testes in two to three irregular rows beneath the dorsal transversal mesenchymatic muscles dorsally to the intestinal branches on each side of the body ( Fig. 18 View FIGURES 18 – 19 ) extend from anterior of the ovaries to just anterior of the pharynx. Sperm ducts, pre-pharyngeally subdivided in two or three ductules, dorsal to ovovitelline ducts, at least two ductules medially displaced; post-pharyngeally right and left sperm ducts form spermiducal vesicles, and open into lateral expansions of the vesicle ( Figs. 20 – 22 View FIGURES 20 – 21 View FIGURES 22 – 25 ).
Prostatic vesicle tubular, mainly extrabulbar, of two portions, a laterally expanded T-shaped ental portion, ventrally located, and a sinuous, almost vertically disposed, unpaired ectal portion, with the distad sinuous part entering the bulbar muscle coat and continuing into the curved ejaculatory duct which opens through the ventral surface of the penis papilla ( Figs. 24, 25 View FIGURES 22 – 25 ). This is a truncate, obliquely disposed ental circular fold which posteriorly communicates with dorsal folds of the male atrium ( Figs. 20, 21 View FIGURES 20 – 21 , 24, 25 View FIGURES 22 – 25 ). Male atrium relatively short ( Table 3 View TABLE 3 ), occupied by dorsal folds of its wall and entally by the penis papilla.
Sperm ducts lined with ciliated cuboidal epithelium; thin muscularis (ca. 4 m thick) mainly comprised of circular fibers. Prostatic vesicle lined with ciliated tall columnar epithelium, receiving abundant erythrophil granular secretion and coarse granular cyanophil secretion, both from secretory cells with bodies lying in mesenchyme, mainly around vesicle. Muscularis of vesicle mainly comprised of circular fibers, thicker in the ental portion (20–30 µm thick) than the ectal portion (10–20 µm).
Ejaculatory duct lined with ciliated columnar epithelium, receiving openings from secretory cells with amorphous, cyanophil secretion and subepithelial bodies as well as from some intra-papillial cells with erythrophil granular secretion. Muscularis of ejaculatory duct (5–10 µm thick) comprised of mixed circular and longitudinal fibers. Penis papilla lined with non-ciliated columnar epithelium with apical xanthophil layer. Three types of secretory cells run longitudinally in the papilla, with numerous openings into the male atrium through its lining epithelium: (1) cells with granular xanthophil secretion; (2) cells with granular erythrophil secretion; and (3) cells with cyanophil amorphous secretion. Erythrophil and cyanophil cells present cell bodies external to common muscle coat; xanthophil cells, intrapapillar, mainly subepithelial cells bodies. Muscularis (10–15 µm) mainly composed of circular fibers with some mixed longitudinal fibers.
Male atrium with the same lining epithelium as the penis papilla. Three types of secretory cells empty through the epithelium: (1) cells with granular xanthophil secretion and subepithelial cell bodies; (2) cells with granular erythrophil secretion; and (3) cells with cyanophil amorphous secretion. Erythrophil and cyanophil cells present cell bodies external to common muscle coat. Muscularis well developed (15–35 µm) of a circular subepithelial layer, underneath by a longitudinal layer.
Ovaries ovoid in shape, measuring 0.4 mm anterior-posteriorly and 0.25 mm dorso-ventrally in the holotype. Ovovitelline ducts emerge dorsally and laterally displaced from the end of anterior third of ovaries, lead backwards immediately dorsal to nerve plate; behind gonopore, ascend posteriorly and medially, to unite dorsally with the female atrium (proflex condition with dorsal approach), to form the common glandular ovovitelline duct. The latter, dorsal to the most ental part of female atrium, recurves anteriorly to communicate with the vagina a terminal dorsally directed diverticulum of female atrium ( Table 3 View TABLE 3 ). Female atrium, oval-elongate in shape, relatively long, and highly folded ( Figs. 20, 21 View FIGURES 20 – 21 , 23 – 25 View FIGURES 22 – 25 ). Length of female atrium, 156% of male atrium length in the holotype ( Table 3 View TABLE 3 ).
Paired ovovitelline ducts lined with ciliated cuboidal epithelium; near to the copulatory apparatus this grades into a ciliated columnar epithelium that also lines the common glandular ovovitelline duct. Paired ovovitelline ducts and common glandular ovovitelline duct with muscularis comprising mixed circular and interposed longitudinal muscle fibres. Abundant shell glands with xanthophil secretion empty into distal third of ascending portion of paired ovovitelline ducts, besides into common glandular ovovitelline duct ( Figs. 20, 21 View FIGURES 20 – 21 , 24, 25 View FIGURES 22 – 25 ).
Female atrium and vagina lined by tall columnar to pseudoestratified epithelium with strongly xanthophil cytoplasm, and exhibiting irregular height and multilayered aspect in some places ( Fig. 25 View FIGURES 22 – 25 ). Entally, epithelial lining of vagina ciliated. Vagina and female atrium receive abundant xanthophil granular and cyanophil amorphous secretions. Cell bodies of cyanophil glands are located between fibers of the atrial stroma or external to the common muscle coat and those of xanthophil cells are internal to the common muscle coat. Muscularis (15–20 µm) composed mainly of circular fibers mixed with some longitudinal fibers.
Gonopore canal posteriorly inclined. Male and female atria with ample communication, without separating folds ( Figs. 20 View FIGURES 20 – 21 , 25 View FIGURES 22 – 25 ). Common muscle coat with circular, longitudinal and oblique fibers, thickest entally and along dorsal wall of male, thinner around female atrium. A stroma with sparse mixed muscle fibres separates the atrial muscularis and common muscle coat.
Vitellaria, situated between intestinal branches, open into the ovovitelline ducts.
Remarks. Vitellaria were well developed in the holotype, although in maturation in paratypes MZU PL.00112 and MZU PL.00113. In paratype MZU PL.00113, length of female atrium relatively to male atrium is shorter (95%, see table 3). Paratypes MZU PL.00113 and MZU PL.00114 were found in copulation.
TABLE 3. Measurements, in milimeter, of specimens of Geoplana carbayoi sp. nov. -: not measured; *: After fixation; ** Specimens with damaged anterior tip (lost or regenerating); DG: distance of gonopore from anterior end; DM: distance of mouth from anterior end; DMG: distance between mouth and gonopore; DPVP: distance between prostatic vesicle and pharyngeal pouch. The numbers given in parentheses represent the position relative to body length.
| Holotype MZUSP PL.01147** | Paratype Paratype MZU PL.00112 MZU PL.00113** | Paratype MZU PL.00114** | Paratype MZU PL.00115** | |
|---|---|---|---|---|
| Maximum length in extension | 130 | 112 91 | 141 | 141 |
| Maximum width in extension | 11 | 12 8 | 15 | 13 |
| Length at rest | 93 | 76 55 | 78 | 70 |
| Width at rest | 15 | 16 12 | 18 | 18 |
| Length* | 104 | 78 66 | 117 | 114 |
| Width* | 15 | 14 12 | 15 | 19 |
| DM* | 62 (60) | 50 43 (64) (65) | 72 (62) | 69 (61) |
| DG* | 75 (72) | 60 52 (77) (79) | 87 (74) | 82 (72) |
| DMG* | 13 | 10 9 | 15 | 13 |
| DPVP* | 3 | 1.8 1.5 | - | - |
| Creeping sole % | 97 | - 91 | - | - |
| Ovaries | 28 (27) | - - | - | - |
| Anteriormost testes | 26 (25) | - - | - | - |
| Posteriormost testes | 56 (54) | - 37 (56) | - | - |
TABLE 4. Cutaneous musculature and body height, in micrometer, in the median region of a transverse section of the pre-pharyngeal region, and ratio of the height of cutaneous musculature to the height of the body (mc: h index) of specimens of Geoplana carbayoi sp. nov. Pre-pharyngeal region of the holotype shows artefacts.
| Holotype | Paratype MZU PL.00112 | |
|---|---|---|
| Dorsal musculature | 44 | 63 |
| Ventral musculature | 55 | 68 |
| Body height | 1790 | 2215 |
| Mc:h (%) | 5 | 6 |
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |