Hemicyclopora celtica, Norman, 1894
|
publication ID |
https://doi.org/ 10.5252/zoosystema2023v45a10 |
|
publication LSID |
urn:lsid:zoobank.org:pub:370E4D0A-FF10-4CAC-AF9F-A1A866FC1BEB |
|
DOI |
https://doi.org/10.5281/zenodo.8057028 |
|
persistent identifier |
https://treatment.plazi.org/id/27282FDA-CBCE-4A9E-812F-9E3CAB2859BF |
|
taxon LSID |
lsid:zoobank.org:act:27282FDA-CBCE-4A9E-812F-9E3CAB2859BF |
|
treatment provided by |
Felipe |
|
scientific name |
Hemicyclopora celtica |
| status |
sp. nov. |
“ Hemicyclopora ” celtica n. sp.
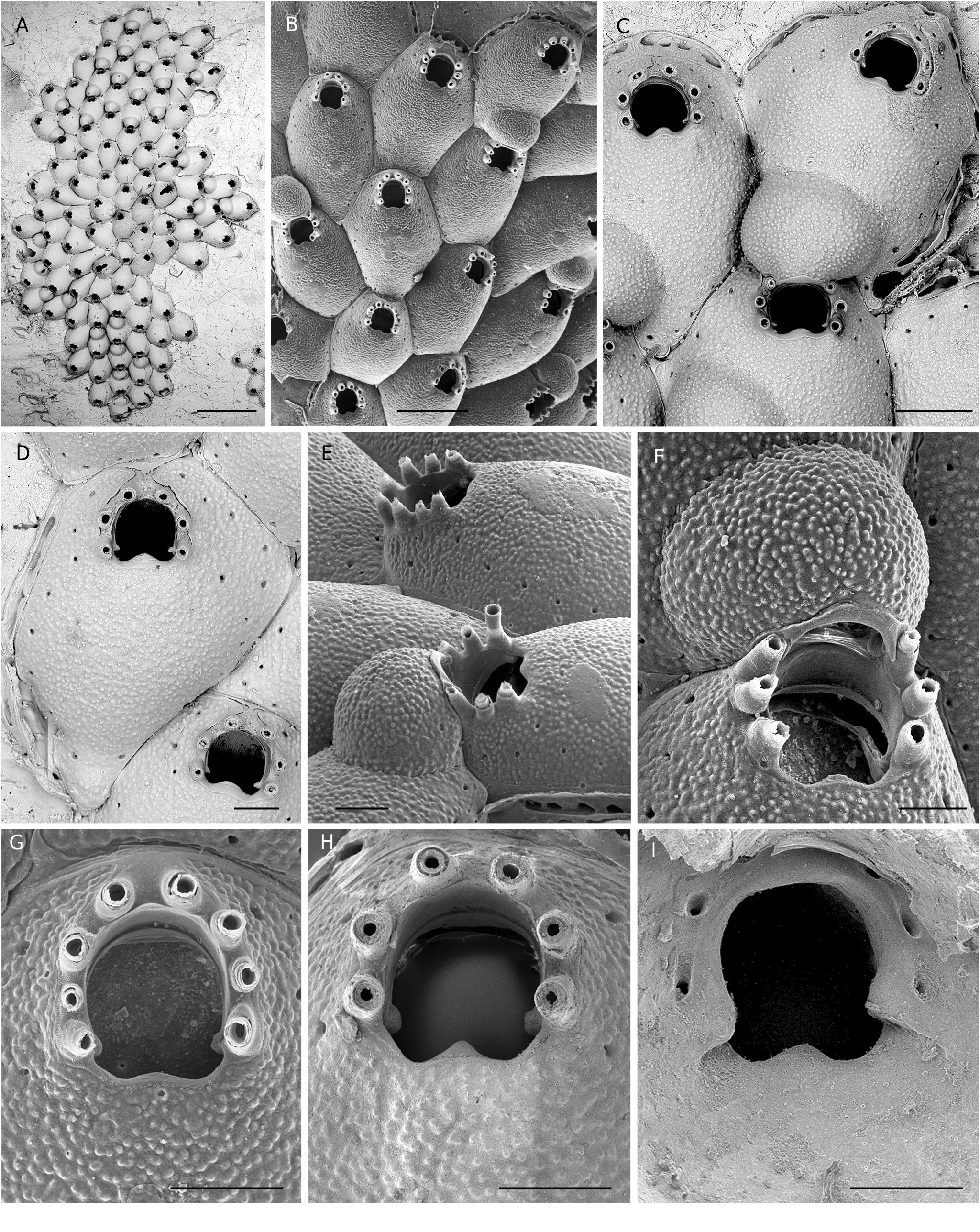
( Fig. 6 View FIG A-I; Tables 1 View TABLE ; 3 View TABLE ; 4 View TABLE )
urn:lsid:zoobank.org:act:27282FDA-CBCE-4A9E-812F-9E3CAB2859BF
TYPE LOCALITY. — Armorican margin, NE Atlantic Ocean.
TYPE MATERIAL. — Holotype. NE Atlantic, France, Armorican margin • 1 large colony, c. 100 autozooids with 15 ovicells; R/V Thalassa ; Stn Z398; 47°36.0’N, 7°16.8’W; 330 m depth; 22.X.1973; on Chlamys shell, together with 1 ancestrula with a single daughter zooid; JGH leg.; Dre; MNHN-IB-2017-778 . GoogleMaps
Paratypes. NE Atlantic, France, Armorican margin • 1 large ovicellate colony, c. 70 autozooids; R / V Thalassa ; Stn Z417, Little Sole Bank, 48°12.0’N, 9°09.5’W; 865 m depth; 24.X.1973; on M. oculata Linnaeus, 1758 ; Dre; JGH leg.; MNHN-IB-2017-779 GoogleMaps • 1 ovicellate colony, coated for SEM examination; R / V Thalassa ; Stn Z435, off Brittany, 48°39.7’N, 09°53.2’W; 1050 m depth; 26.X.1973; with H. polita on Desmophyllum pertusum (Linnaeus, 1759) ; Dre; JGH leg.; MNHN-IB-2017-780 GoogleMaps • 1 ovicellate colony, c. 65 autozooids with 26 ovicells; R / V Thalassa ; Stn Z398; same data as holotype; on shell; PMC. B35.5.5.2021.
Ireland • 3 small colonies; Trawler Heliotrope , Porcupine Seabight, 51°30’N, 11°30’W; 1000 m depth; II.1977: on shells and coral skeleton; Dre; JGH leg.; MNHN-IB-2017-781 GoogleMaps .
OTHER MATERIAL EXAMINED. — NE Atlantic – France, Armorican margin • 1 colony; R / V Thalassa ; Stn Z392, Armorican Margin; 47°34.9’N, 7°01.3’W; 390 m depth; 21.X.1973; MNHN GoogleMaps • 3 colonies; R / V Thalassa ; Stn Z398, same data as holotype • 1 colony; R / V Thalassa ; Stn Z402, 47°39.5’N, 07°28.5’W; 450 m depth; 22.X.1973; MNHN GoogleMaps • 1 colony; R / V Thalassa ; Stn Z427; 48°27’N, 09°48.4’W; 330 m depth; 25.X.1973; on D. pertusum ; MNHN GoogleMaps • 1 colony; R / V Thalassa ; Stn Z417; on M. oculata ; same data as paratype MNHN-IB-2017-779 • 1 colony; R / V Thalassa ; Stn Z435; 1050 m depth; on D. pertusum ; same data as paratype MNHN-IB-2017-780.
ETYMOLOGY. — Latin adjective, feminine of celticus, in reference to the frequency of this species in the Celtic Sea.
DIAGNOSIS. — Autozooids bulged, relatively large, frontal shield with small, rounded granules, small marginal pores. Orifice terminal to subterminal, condyles prominent, with blunt tips, proximal edge convex, with a narrow gymnocystal rim, without proximal inner thickening. Oral spines typically eight, but sometimes six or seven in non-ovicellate zooids, always six in ovicellate zooids. Ovicells with a narrow gymnocystal proximal rim, lying on the distal, ooecium-builder autozooid. Ancestrula with opesia, cryptocyst and gymnocyst equally extended along central long axis, 11, 12 or 13 spines.
DESCRIPTION
Colony encrusting, unilaminar, small to medium-sized. Autozooids distinctly separated by deep grooves, laid out in quincunx, relatively large, oval to hexagonal, the width often much variable; frontal shield convex, evenly covered by small, rounded granules; marginal pores small (10-15 µm), arranged in a single row which becomes double laterally to the orifice ( Fig. 6 View FIG C-E). Basal pore-chambers oval to elongated, grouped by four to five in elongated windows, two disto-lateral and one distal ( Fig. 6 View FIG C-E). Distal wall subvertical ( Fig. 6 View FIG B-E). Orifice terminal or subterminal, as long as wide or slightly longer than wide in non-ovicellate zooids ( Fig. 6C, D, G, H View FIG ), wider in ovicellate zooids ( Fig. 6C View FIG ); proximal edge often clearly convex, i.e., in shape of a parabola ( Fig. 6C, D, H View FIG ), but sometimes nearly straight ( Fig. 6B, G View FIG ), without umbo or distinct cryptocystal thickening; primary orifice with prominent condyles, medium-sized, with blunt or triangular tips ( Fig. 6D, G, H View FIG ), inner side of the proximal edge smooth, without any gymnocystal thickening ( Fig. 6I View FIG ). Oral spines long, slender, with acute tip, seemingly composed of two jointed segments, peristomial bases conical and thick, eight in a majority of non-ovicellate zooids, but also seven or six (see below), always six in ovicellate zooids ( Fig. 6 View FIG ). Ovicell adnate on the distal ooecium-producing daughter autozooid, apparently acleithral, endooecial surface granular as the frontal shield, with a narrow, smooth gymnocystal thickening bordering the proximal edge of the orifice ( Fig. 6C, E, F View FIG ). Ancestrula with six spines distally around the opesia and five, six or seven spines proximally around the cryptocyst; gymnocyst well developed (30-40% of total length along the proximo-distal axis).
REMARKS
Morphological features
“ Hemicyclopora ” celtica n. sp. is characterized by the following features: 1) orifice with a distally-curved proximal edge whose convexity is more or less pronounced, and without umbo; 2) condyles prominent with triangular or rounded tips; 3) inner side of proximal part of primary orifice with smooth and flat surface; 4) invariably six spines in ovicellate zooids (117 ovicells examined); 5) ovicell recumbent on the proximal part of the frontal shield of the distally adjacent autozooid into which it is integrated; 6) ovicell opening edged with a smooth narrow gymnocystal rim, apparently acleithral, as suggested by examination of the holotype with a stereomicroscope and SEM photos of bleached specimens ( Fig. 6F View FIG ); however, accurate identification of the ovicell closure requires examination of living colonies and anatomical studies ( Ostrovsky 2013); and 7) small marginal pores ( Table 3 View TABLE ). The number of spines in non-ovicellate zooids is usually eight but can be lower ( Fig. 6 View FIG B-E). In 160 non-ovicellate zooids from 12 colonies (7 localities, 330-1050 m) the number of spines was eight (45%), seven (39%) or six (16%). This variability is apparently not induced by factors of the microenvironment. This assertion is substantiated by colonies of similar size and condition occurring on the same fragment of coral skeleton (two cases: Thalassa Z402 and Z417) which presented inverse ranking in their proportions of spine numbers. The occurrence of eight spines in non-ovicellate zooids is assumed to be a fundamental trait of “ H.” celtica n. sp. while a lower number, six or seven spines, would result from an aborted development of the ovicell. The proportion of ovicellate zooids per colony can be high ( Fig. 6A View FIG ), but is in general moderate (about 8-17%; mean = 11 ± 4%). Another source of morphological variability in “ H.” celtica n. sp. is the shape of autozooids due to the great range of the autozooid width ( Table 1 View TABLE ). This feature is reflected by the value of the coefficient of variation (SD × 100/X), which is higher for width than for length (18% vs 11%).
Taxonomic issues
“ Hemicyclopora ” celtica n. sp. is morphologically very close to Escharella lopezfei Souto, Berning & Ostrovsky, 2016 , from the Galicia Bank (NE Atlantic). These species display several similar external features: same aspect of the frontal shield with small rounded granules and small marginal pores, same layout of the pore-chambers, proximal edge of the orifice and condyles similarly shaped, usually eight oral spines in non-ovicellate zooids, ovicells associated to the frontal shield of a distal autozooid. Obviously, the series of traits shared by these two entities raises the problem of their specific and generic assignment. The decision to separate our material from E. lopezfei at both species and genus ranks was justified by the conjunction of: 1) the constant difference in the number of oral spines in maternal zooids (always six in “ H”. celtica n. sp. and eight in E. lopezfei ), verified in numerous colonies of “ H. ” celtica n. sp.; 2) the absence in the internal side of the orifice of “ H”. celtica n. sp., below its convex edge and above the level of condyles, of any thicknening forming a lyrula similar to the triangular denticle recorded in E. lopezfei and E. praealta ( Calvet, 1907) , a closely related species according to Souto et al. (2016); and 3) the distribution of these two entities in two distant geographical areas. Precise information on the shape of this denticle in E. praealta is provided by López de la Cuadra & Garcia Gómez (1993: fig. 2; 2001: fig. 1D-F) and unpublished SEM pictures of specimens of this species from Mediterranean cryptic habitats kept in our collection (JGH & AR). Undoubtedly, the structure of the orifice of E. praealta (and thus of E. lopezfei ) differs from that of “ H. ” celtica n. sp. As stressed by Pica et al. (2022), “subtle differences are often considered species-specific” in Bryozoa according to the modern species taxon concept. Therefore, considering the lack of denticle or thickening in the orifice ( Fig. 6I View FIG ) which could play the role of a lyrula ( Berning et al. 2014) and the uncertainty concerning the type of ovicell closure and its value as discriminating feature, “ H”. celtica n. sp. was arbitrarily attributed to the genus Hemicyclopora . Obviously, this species constitutes a ‘borderline case’, such as the entities forming the species complex similis (see below). Both cases challenge the distinction between the genera Hemicyclopora and Escharella .
The proximal edge of the orifice of “ H”. celtica n. sp. can be covered by a narrow rim of smooth gymnocystal calcification when it is convex ( Fig. 6C, D, H View FIG ), as in E. lopezfei ( Souto et al. 2016: figs 78-79). However, this feature is not constant in “ H. ” celtica n. sp., and the whole poster edge can be covered by the secondary cryptocystal calcification of the frontal shield ( Fig. 6E, G View FIG ). Moreover, the convexity of the poster edge is variable and can be insignificant ( Fig. 6B View FIG ). When visible in frontal view, the gymnocystal rim of the convex poster is clearly continuous with the gymnocystal frame of the orifice, including the spines, and often remains visible distally, between the distalmost pair of spines ( Fig. 6D View FIG ). This structure indicates more a deficiency in cryptocystal calcification than the emergence of a lyrula-like denticle with a peristomial position. Thus, pending molecular analyses providing a clarification of the phylogenetic relationships between Hemicyclopora and Escharella , this new species is conditionally left in Hemicyclopora .
“ Hemicyclopora ” celtica n. sp. resembles “ H”. pytheasi n. sp. (see below) in having a similar orifice shape and eight oral spines in non-ovicellate autozooids ( Figs 6B, G View FIG ; 9F View FIG ) but they clearly differ in the shape of the condyles and the type of ovicell (see below).
HABITAT DISTRIBUTION
“ Hemicyclopora ” celtica n. sp. is a deep-water species found in seven stations ranging from 330 m to 1050 m depth, mostly located close to or along the shelf break. Colonies encrusted shells, biogenic debris and were frequent on skeletons of ‘cold-water’ corals ( M. oculata Linnaeus, 1758 , D. pertusum (Linnaeus, 1759)) . These fragmented coral skeletons indicate the proximity of banks built by these large branching scleractinians along the edge of the continental shelf where a strong thermocline is established and currents bring nutrientrich waters ( White & Dorschel 2010). In the three deepest stations ( Thalassa Z417, Z435, Heliotrope: 865-1050 m), “ H”. celtica n. sp. co-occurred with H. polita , often on the same fragment of coral skeleton.
GEOGRAPHICAL DISTRIBUTION
“ Hemicyclopora ” celtica n. sp. was recorded in the northeast Atlantic from the Armorican margin to west Ireland ( Table 3 View TABLE ). However, its actual distribution is most likely wider, particularly in deep-water locations of the northern Atlantic.
| R |
Departamento de Geologia, Universidad de Chile |
| V |
Royal British Columbia Museum - Herbarium |
| MNHN |
Museum National d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Lepralielloidea |
|
Family |
|
|
Genus |