Isophya bumerangoides, Sevgili, Hasan, Demirsoy, Ali & Çiplak, Battal, 2012
|
publication ID |
https://doi.org/ 10.5281/zenodo.209689 |
|
DOI |
https://doi.org/10.5281/zenodo.5659977 |
|
persistent identifier |
https://treatment.plazi.org/id/CD62BB35-FF8F-3043-FF55-C5EEFE79C724 |
|
treatment provided by |
Plazi |
|
scientific name |
Isophya bumerangoides |
| status |
sp. nov. |
Isophya bumerangoides , new species
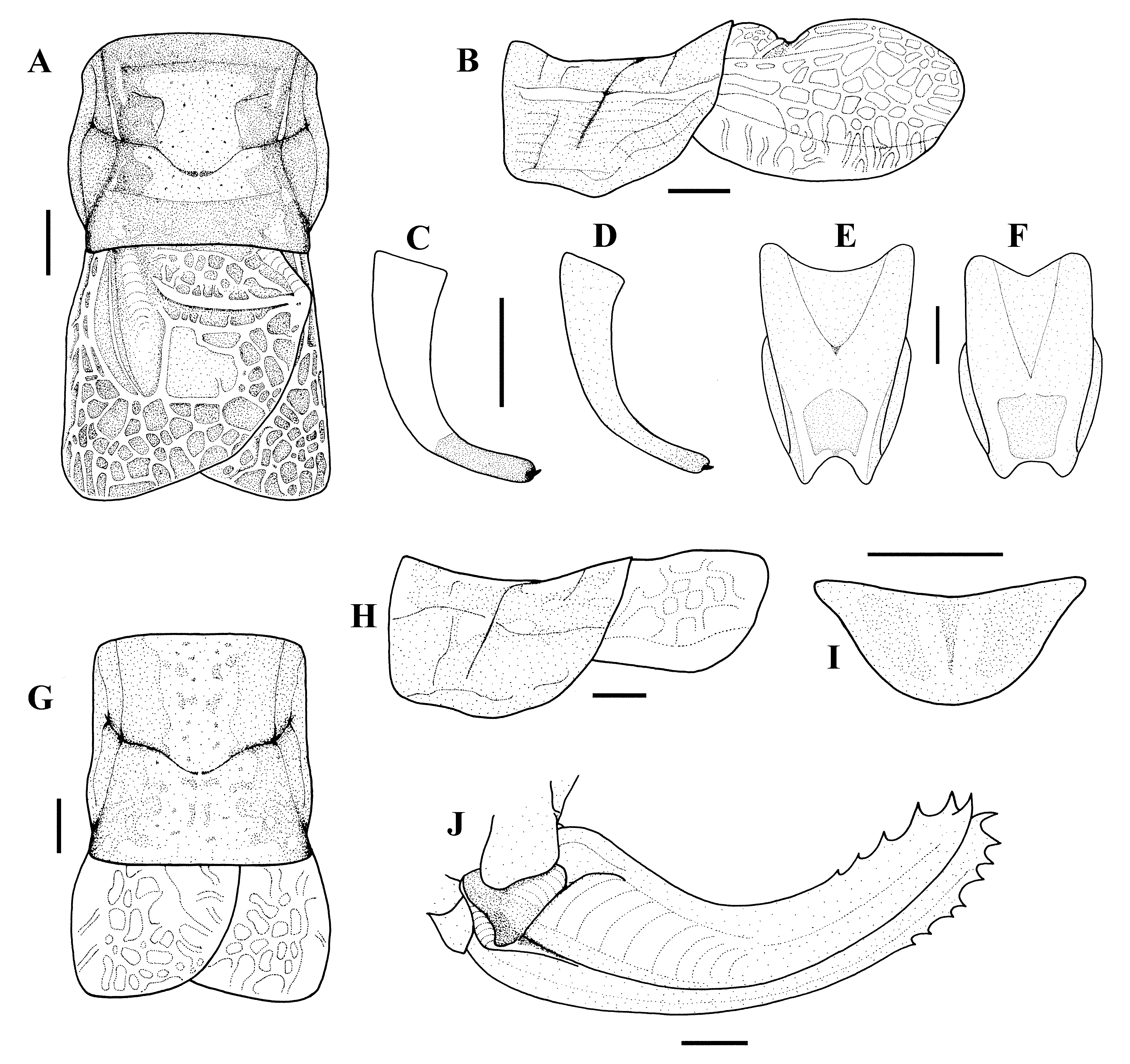
Figs. 1–11 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4. I View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 View FIGURE 11 (including bioacoustic plates)
Material examined: Holotype—Male: TR-Giresun- Keşap, 1951, 3 (Coll. Ankara Zirai Mücadele Enstitüs). Paratypes, the same locality, 433, 2ƤƤ ( HUZOM); -Keşap, Karabulduk, 13.06.2010, 1533, 2ƤƤ (coll. H. Sevgili & E. Sevgili, in alcohol, CHS).
Etymology. Specific name derived from shape of the male cerci. Turkish bumerang— in English boomerang; - oides —like, or similar to boomerang.
Male: Fastigium small with distinct groove, convergent, narrower than half scapus on apex. General appearance of pronotum as saddle, anterior and posterior margin of the pronotum almost straight, metazona clearly wider than prozona and laterally depressed in mesozona in dorsally ( Figs. 1 View FIGURE 1 A, 2A, B, D); middle of transverse groove situated behind middle of pronotum; in lateral wiev dorsal side of the pronotum distinctly concave, prozona slightly but metazona clearly raised, ventral margin of paranota sligthly convex ( Fig 1 View FIGURE 1 B; 2A, B, D). Tegmina longer than pronotum ( Fig. 1 View FIGURE 1 B, 2A, B, D and Table 1 View TABLE 1 ) with striking reticulated, right margin of left tegmen slightly protruding where around proximal part of Cu2; Cu2 narrower than third antennal segment and about 2/3 times longer than posterior margin of pronotum, speculum rectangular. Hind femora without spine ventrally, about four times longer than pronotum.
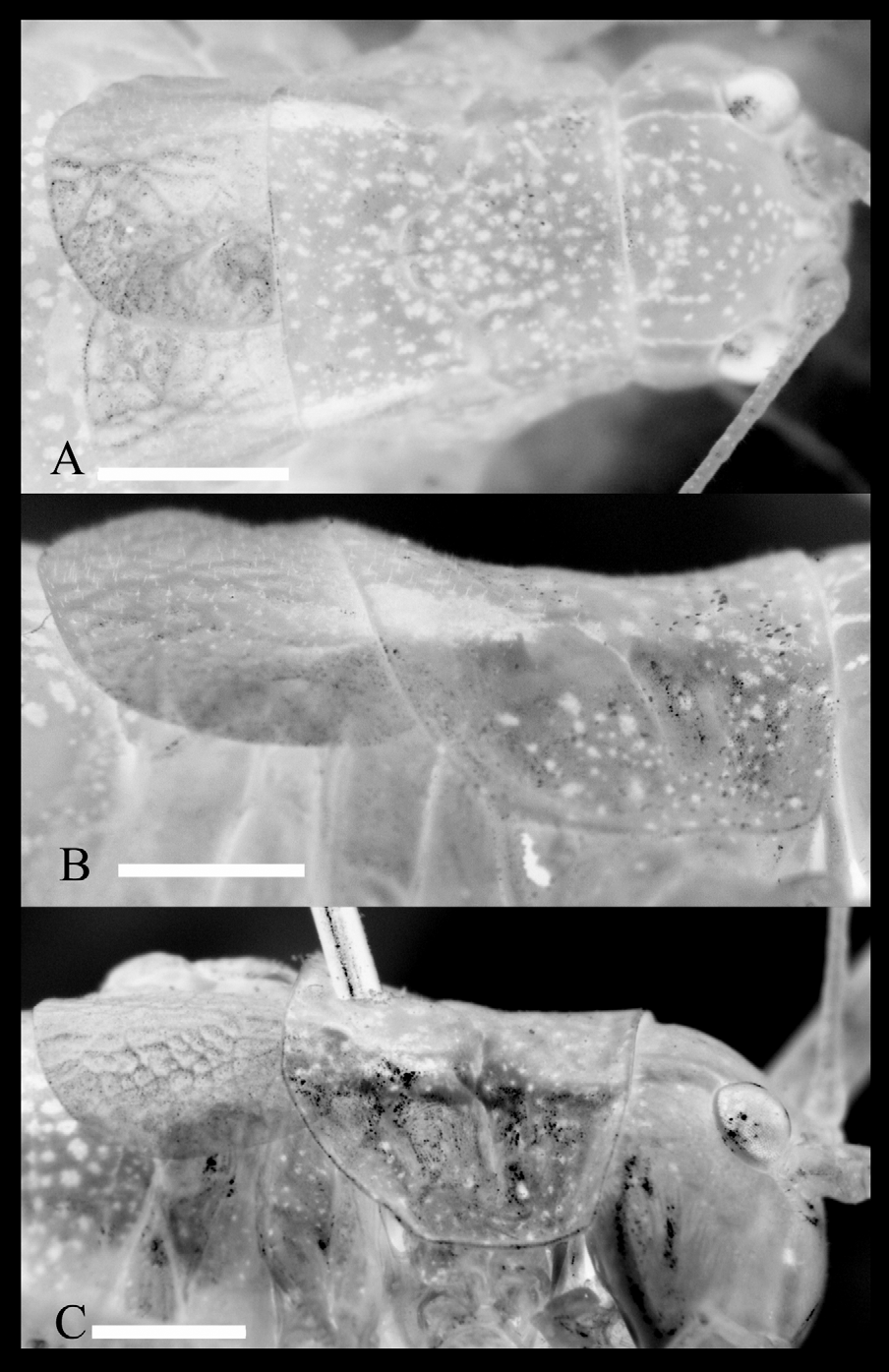
Stridulatory file ( Fig. 3 View FIGURE 3 A, C, D, G): Left tegmina with about 85–98 teeth, reaching posterior margin of tegmina; in ventral view arcuate; shortest distance between proximal and distal most tooth about 2.43 mm.
Epiproct wide, almost twice as wide as long. Cercus ( Fig. 1 View FIGURE 1 C, D) slender, slightly curved at basal 1/3, strongly curved but slightly tapering towards the apex. Apex not pointed, ending a distinct denticle. Subgenital plate ( Figs. 1 View FIGURE 1 E, F; 2A1, B1, D1) swollen, slightly narrowed apically, posterior margin with wide but slightly notch.
(mm; min–max (mean±SD))
Female: Fastigium as wide as half of scapus at the base, slightly wider than male’s, lateral margins convergent, longitudionally impressed above. Pronotal disk almost cylindrical, slightly wider in metazona, posterior margin straight, in profile dorsal surface of pronotum slighlt concave; ventral edges of paranota broadly convex ( Figs. 1 View FIGURE 1 G, H; 4A, B). Tegmina longer than half length of pronotum, extending to beyond middle of first tergite, with reticulate venations ( Figs. 1 View FIGURE 1 G; 4A, B). Hind femur about 4.2 times pronotal length.
Cerci robust, short, extending to beyond posterior edge of epiproct. Subgenital plate broadly half round, without protruding ( Fig. 1 View FIGURE 1 I). Ovipositor ( Figs. 1 View FIGURE 1 J, 5A) gradually upcurved, 2.63 times longer than pronotum, upper margin with 6–7, lower margin with 7–9 denticles. Gonangulum slightly swollen, hind edge relatively straight. Lamella strong and folded, strongly impressed dorsally forming with gonangulum a distinct pit ( Fig. 5 View FIGURE 5 A, A1).
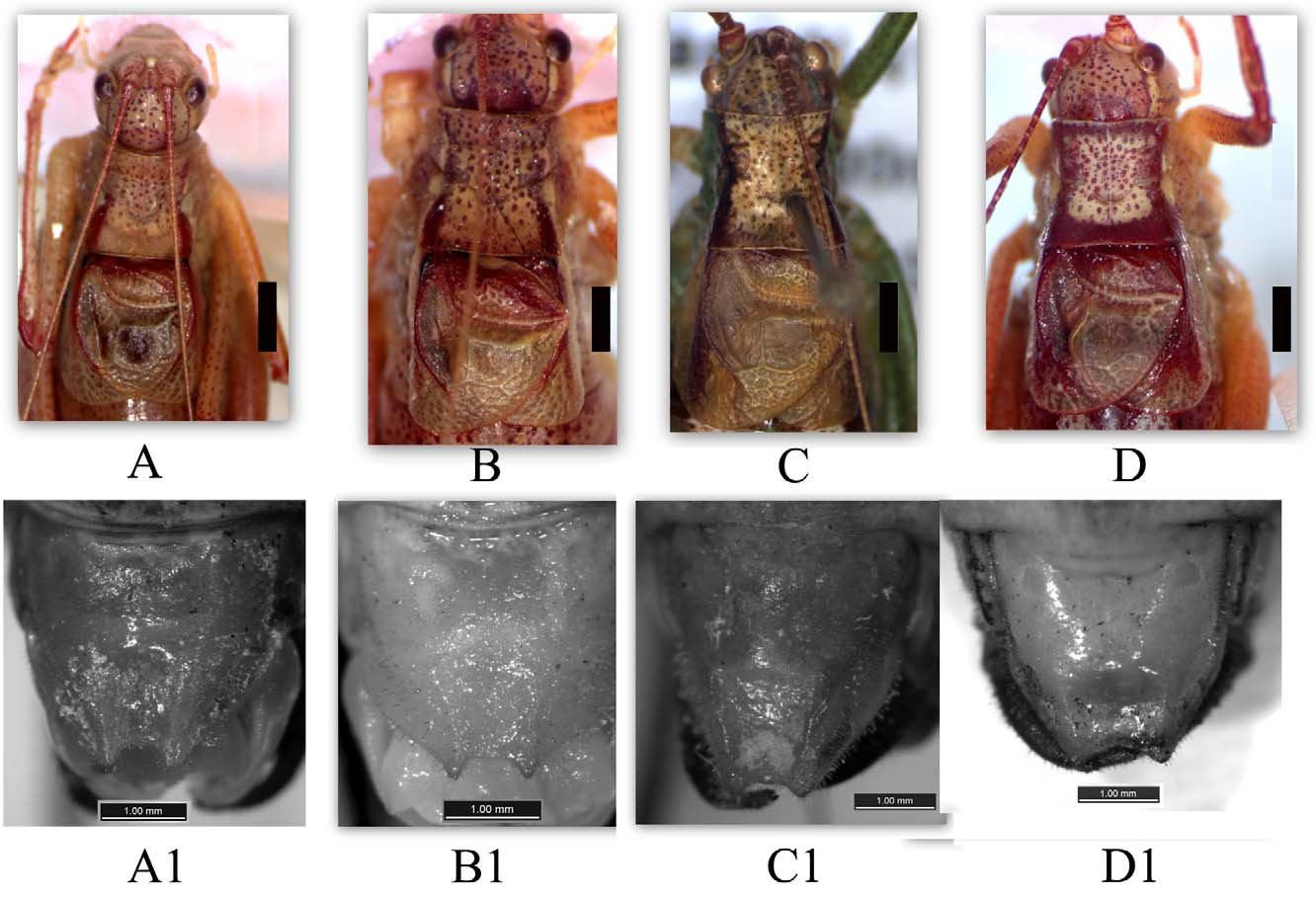
Coloration: Like most species within the genus Isophya , the species is polymorphic for coloration. There are two or three color patterns, with dark, dark-green and pale color morphs in males ( Fig. 6 View FIGURE 6 A–D). These morphs can be found inhabit the same habitats. Females can similarly be classified according to color pattern, but the variation is not as distinct as in males, like in I. rizeensis (Sevgili, 2003; Saġlam & Çaġlar, 2007).
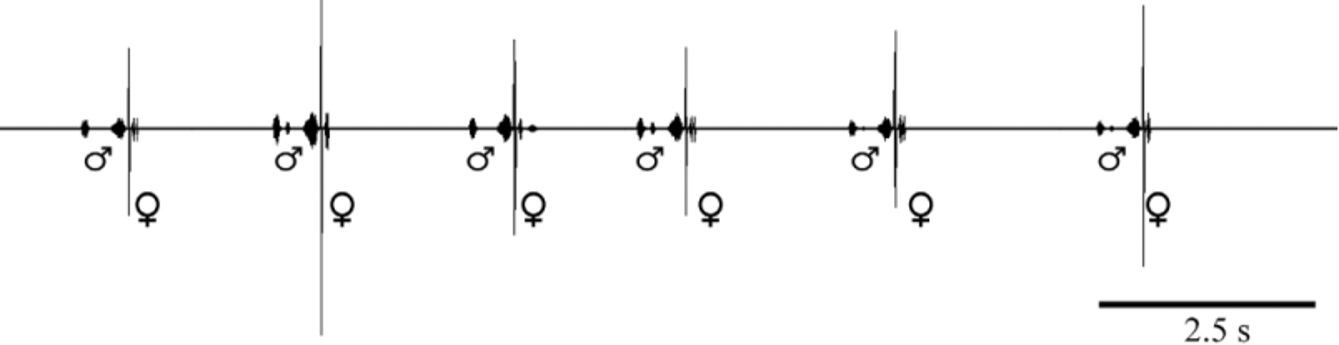
Bioacoustics: 14 males recorded at 28 ºC. Males produce their calling song by tegmino-tegminal stridulation. The male calling song produced mainly in the evening and at night.
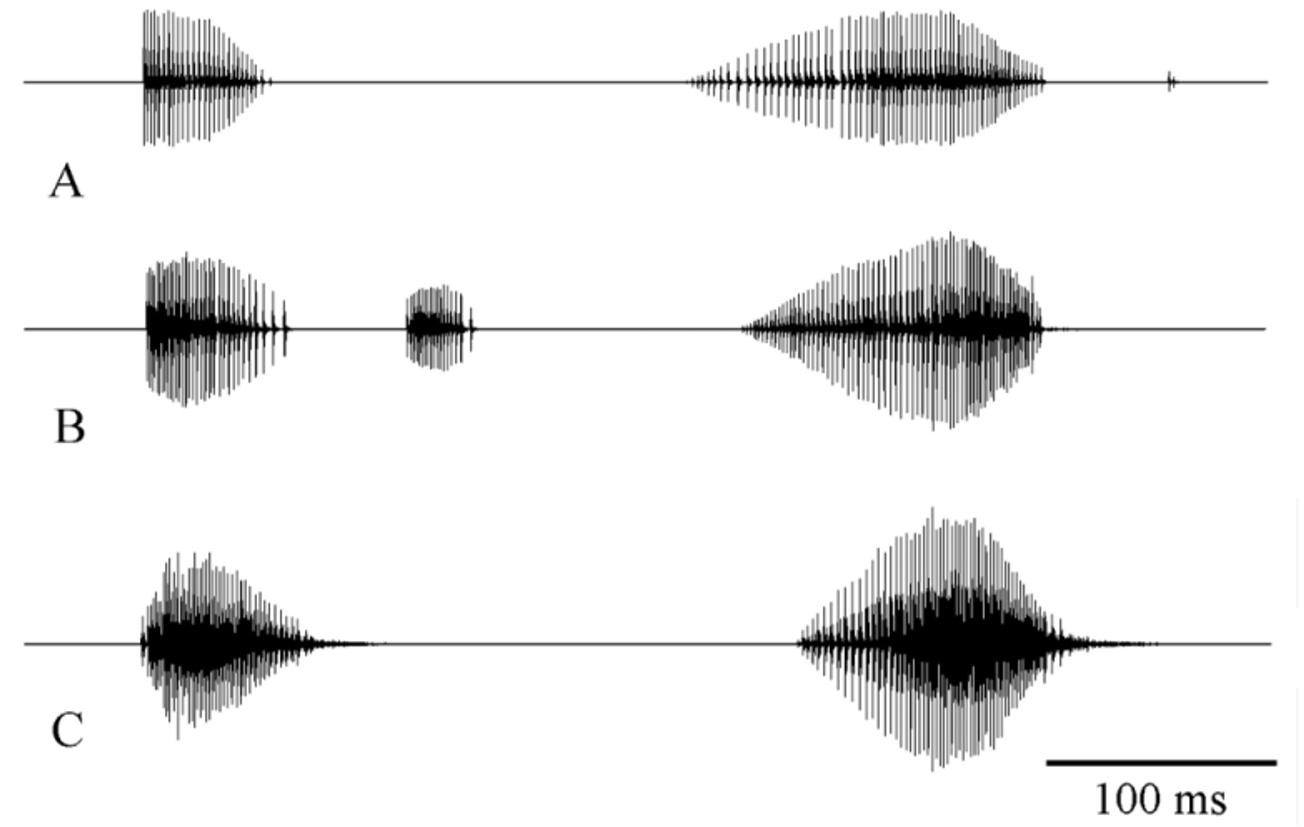
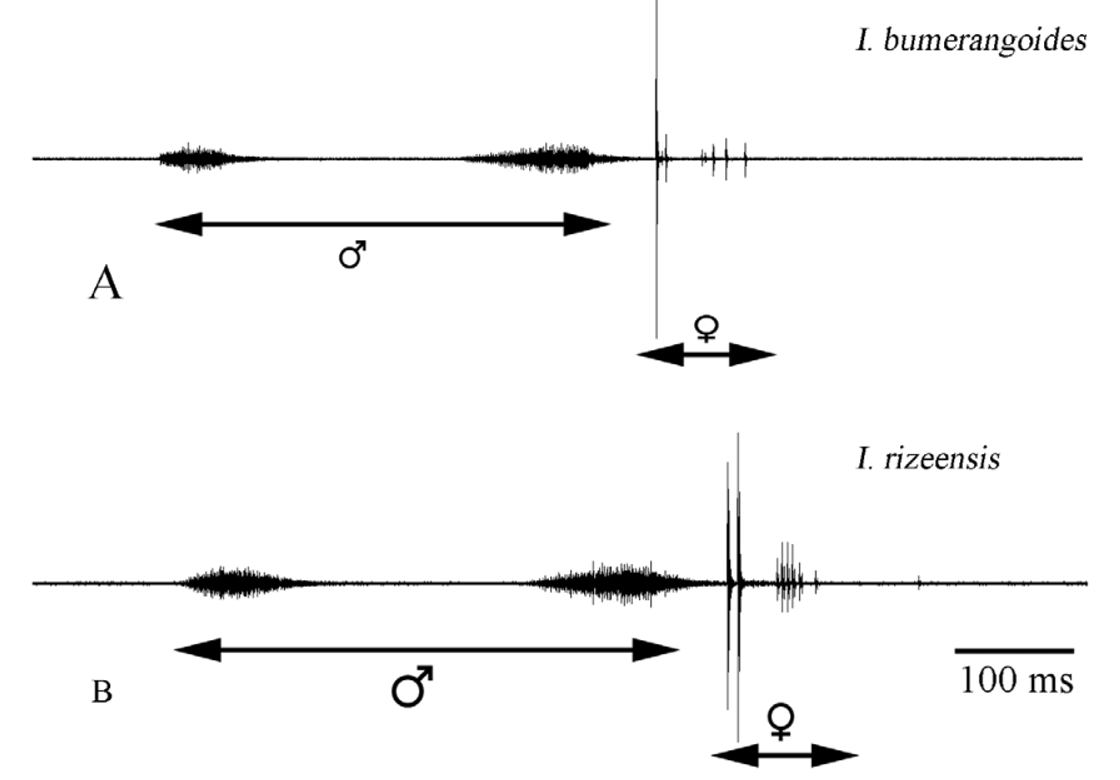
Male calling song: The song consists of two distinct syllables ( Fig. 7 View FIGURE 7 A, B), recorded at 28ºC. The song can be formulated as (A1…a1......B), (A1…a1.B+click) or sometimes (A........B+click), repeated at very variable intervals (about mean: 59 s) with rather irregular repetition rate of 5 -24 calling songs per 5 minutes. Each song lasts for about 288–527 ms (mean: 372±36.35 SD, n= 164).
A1 syllable consists of a series 16–84 (mean: 4311 SD, n= 164) impulses (duration mean: 59 ms 6.5 SD) with increasing in amplitude at the beginning in some recordings. The signal amplitude in A1 and a1 syllables slightly increases at the beginning and decreases from the middle part to the last impulses. The last syllable (B) is different from A1 and a1, consists of 46–92 impulses (mean: 697.6, n= 164), their signal amplitudes are distinctly crescendo and slightly decrescendo in the last impulses. The last syllable lasts 90–155 ms (mean: 118.28±13.66SD, n= 164).
In some calling songs, there is minor syllable, is smaller than A1 and has 5–35 impulses (a1), last about 8–42 ms. The after-clicks follow the last syllable after a short interval of 29–97 ms (mean: 56.23±12.37SD, n= 21) with a few impulses in some calling songs.
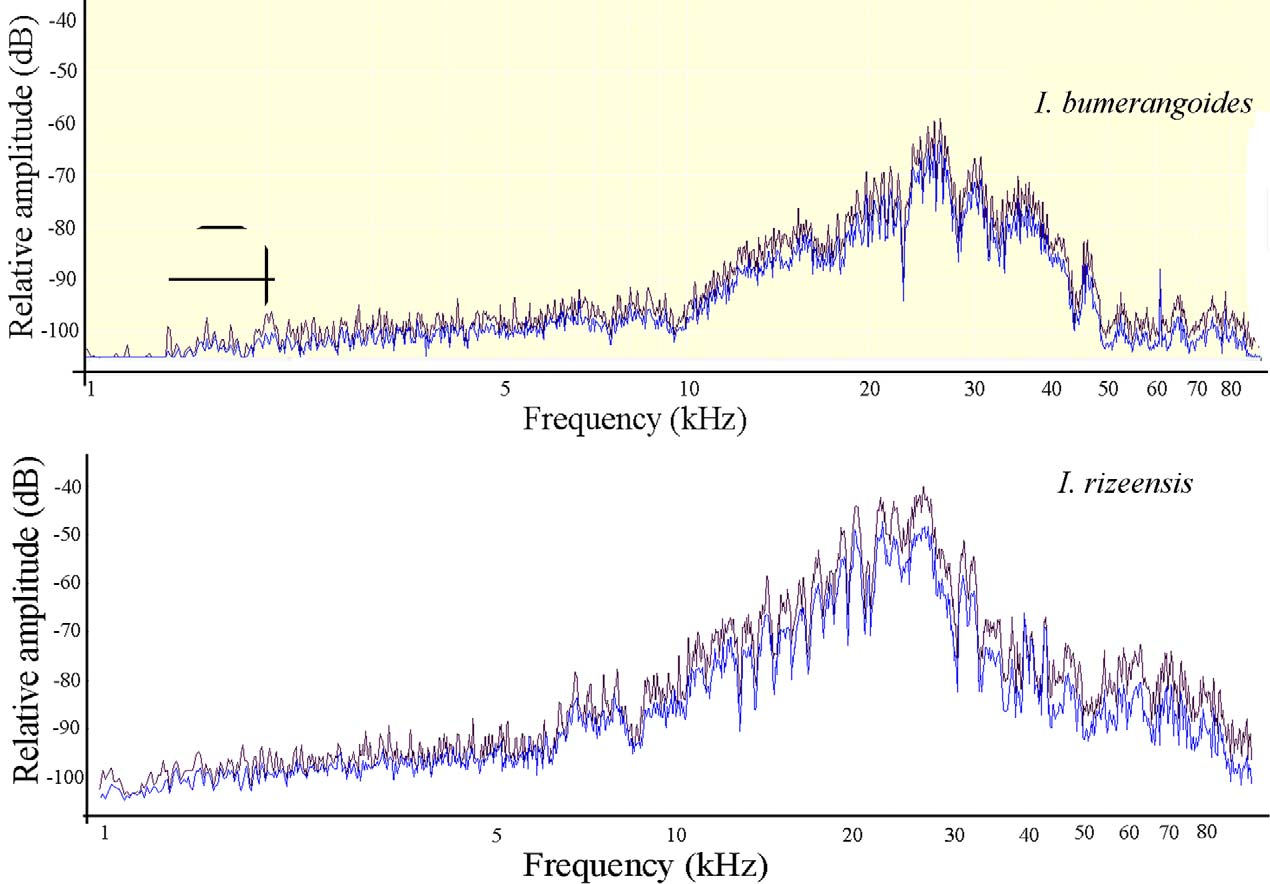
The male song has a wide band frequency spectrum with intensive components between 5–90 kHz ( Fig. 8 View FIGURE 8 ). Within that range three intensity peaks can be observed: a less intensive one between about 10-50 kHz and a more intensive range between about 19–30 kHz (peak frequence mean: 23.48 (min.-max. 15.73–28.92).
Female song: Females’ response as mating acceptance with the singing male is a series of a few impulse group produced after the last syllable (B) with short major and minor impulses ( Fig. 9 View FIGURE 9 ). The duration of between male song and females’ response song varied between 15–187 ms (mean: 39.35±24.22SD, n= 43 from two females, at 28?C). The beginning group of the response song contains usually major 1–3 impulses, the second group contains 1–7 minor impulses ( Fig. 10 View FIGURE 10 A). Whole female song lasts about 68 ms (n= 45). The carrier wave has its most intensive components between 21–28 kHz (mean: 24. 85, n= 19) ( Fig. 11 View FIGURE 11 ).
Distribution: Known only from type locality (Keşap, Giresun).
Remarks: The species is characterized by the shape of cerci in the male. Our results show that the new species can be distinguished from the closely related species, I. reticulata , I. sureyai , I. rizeensis and I. redtenbacheri confidently on the basis of the examined morphometric and bioacoustic characters (most of them own unpublished data). In the examined male specimens we found significant differences among fastigium, tegmina, cerci and subgenital plate. We have also found some differences among the stridulatory files; however, the stridulatory file varies considerably in these species ( I. rizeensis 77–103 (new data)); I. redtenbacheri about 75; I. sureyai 115–145; I. reticulata 102–123, own unpublished data). The arrangement pattern of stridulatory pegs also differ for the species (compared with I. redtenbacheri Fig. 3 View FIGURE 3 B, E, F, H and for I. rizeensis see Sevgili, 2003).
I. bumerangoides is more closely related to I. rizeensis Sevgili, 2003 and I. redtenbacheri Adelung, 1907 in terms of general appearance of pronotum ( Fig. 2 View FIGURE 2 A–D) male cerci and structure of male subgenital plate ( Fig. 2 View FIGURE 2 A1- D1). Although these species look quite similar they display significant differences in important diagnostic traits such as body length ( I. rizeensis is distinctly bigger than I. bumerangoides ; see Table 1 View TABLE 1 ), male-female fastigium, tegmina, male cerci, subgenital plates, structure of female lamella and gonangulum. This new species is distinguished from I. rizeensis by its smaller body size, more convergent fastigium with a broad deep dorsal cavity, almost short and swollen subgenital plate in the male and, shorter ovipositor. Moreover, in the shape of lamella and gonangulum. I. bumerangoides is clearly different from I. redtenbacheri by its less curved male cerci, different morphology of ovipositor, lamella and gonangulum ( Figs. 5 View FIGURE 5 A-A1, B-B1). Although the number of stridulatory pegs of both species is similar, there are some differences in the arrangement pattern of them. For many Isophya species, the number of stridulatory pegs can be used as taxonomic character ( Heller, 1988; Ragge & Reynolds, 1988; Sevgili, 2004; Orci et al. 2010).
The calling songs of other closely related species, except I. redtenbacheri , are similar and contain at least two syllables, but the amplitude modulation, number of impulses in the syllables, duration of syllables are variable (HS unpublished data). The males of I. bumerangoides and I. redtenbacheri may be easily distinguished from each other on the basis of the calling song. In I. redtenbacheri the male calling song consists of single syllable that is irregularly repeated (HS unpublished data). The spectral characteristics of the calling songs of I. bumerangoides are very similar to I. rizeensis ( Fig. 7 View FIGURE 7 A–C). Spectrogram analysis of I. rizeensis revealed the main frequency in a syllable range between 14 and43 kHz with a maximum range at about 19-27 (peak frequency mean: 21.38, n= 38) kHz ( Fig. 8 View FIGURE 8 ).
The female emitted their response songs after the last syllables (B) of the male calling song. The females’ song pattern and frequency spectrum of I. bumerangoides are similar to I. rizeensis ( Figs. 9 View FIGURE 9 , 10 View FIGURE 10 and 11 View FIGURE 11 ). Like other phaneropterine females, I. bumerangoides females respond with a short latency and a defined acoustic signal to the song of a conspecific male and timing of reply is consistent. The singing female system relatively common amongs members of the phaneropterine species as in Isophya ( Heller and Helversen, 1986; Robinson, 1990; Heller, 1990; Spooner, 1995). Many female Isophya species produces a sound (usually a series impulses or short click) in response to the male call and our observation show that she remains still whilst the male moves towards her. In both I. bumerangoides and I. rizeensis , there is little variation in female response songs. Isophya species can communicate within short and middle range, so in this system they should use the high-frequency signals (HS unpublished data; Heller and Helversen, 1986). The new species sings in the region of about 20–30 kHz, as in other some Isophya and Poecilimon species.
The new species, I. bumerangoides , is not a single species, but a species group ( I. amplipennis group) including at least ten species (such as I. reticulata , I. rizeensis , I. redtenbacheri , I. sureyai , I. rodsjankoi , I. savignyi , I. uludaghensis , I. speciosa ) ( Sevgili, 2004, HS unpublished data). The amplipennis group can be distinguished from other Isophya species by some morphological and bioacoustical characters such as morphology of pronotum and elytra, mainly male calling songs with two macro syllables and microsyllables between the main macrosyllables in some members. Our results support the validity of the specific status of this narrow range, vulnerable bush-cricket. In conclusion, it is likely that divergence within this group is quite recent and not yet completed as in many Isophya species ( Sevgili, 2004). Therefore, the molecular studies would be needed to explain the evolutionary relationships within the Isophya species.
TABLE 1. Comparision of I. bumerangoides sp. n. with I. rizeensis based on various body measurements. Species / Measurements I. bumerangoides sp. n. I. rizeensis Sevgili, 2003
| Male (n=18) | Female (n= 11) | Male (n=50) | Female (n= 30) | |
|---|---|---|---|---|
| Body length | 16.00–23.91 (21.19±2.16) | 17.00–22.34 (19.65±2.53) | 17.70–27.57 (24.60±3.39) | 19.00–27.05 (23.91±3.10) |
| Pronotum | 2.96–3.84 (3.37±0.27) | 3.30–4.34 (3.95±0.42) | 3.10–4.20 (3.77±0.31) | 3.80–4.80 (4.25±0.33) |
| Tegmina Hindfemur Ovipositor | 3.30–4.66 (4.16±0.35) 12.65–14.94 (14.13±0.61) -- | 1.80–3.53 (2.67±0.62) 14.45–16.20 (15.10±0.69) 7.80–8.31 (8.12±0.21) | 3.90–5.32 (4.82±0.44) 14.40–17.96 (16.39±1.24) -- | 2.40–3.50 (3.07±0.40) 16.00–19.20 (17.61±1.06) 9.00–12.00 (10.73±1.03) |
| Tegmina/Pronotum | 1.26 | 0.74 | 1.30 | 0.77 |
| Body length/Hindfemur Hindfemur/Ovipositor | 1.56 -- | 1.53 1.78 | 1.58 -- | 1.49 1.61 |
| Ovipositor/Pronotum | -- | 1.94 | -- | 2.63 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Phaneropterinae |
|
Genus |
Isophya bumerangoides
| Sevgili, Hasan, Demirsoy, Ali & Çiplak, Battal 2012 |
I. rizeensis
| Sevgili 2003 |
I. redtenbacheri
| Adelung 1907 |