Lerista parameles, Amey & Couper & Wilmer, 2019
|
publication ID |
https://doi.org/10.11646/zootaxa.4577.3.3 |
|
publication LSID |
lsid:zoobank.org:pub:CA0CE52C-49EE-4F8C-8DD4-6A3297D982BB |
|
DOI |
https://doi.org/10.5281/zenodo.5927493 |
|
persistent identifier |
https://treatment.plazi.org/id/E3C2475F-F027-4EBB-9B68-CBCE2ED9A30A |
|
taxon LSID |
lsid:zoobank.org:act:E3C2475F-F027-4EBB-9B68-CBCE2ED9A30A |
|
treatment provided by |
Plazi |
|
scientific name |
Lerista parameles |
| status |
sp. nov. |
Lerista parameles sp. nov.
Chillagoe Fine-lined Slider
( Figs. 4 View FIGURE 4 , 10 View FIGURE 10 & 11 View FIGURE 11 )
ZooBank ID No. E3C2475F-F027-4EBB-9B68-CBCE2ED9A30A
Holotype. QM J95783 View Materials , Savanna Way, S Almaden, NEQ ( 17°24'04"S, 144°38'52"E), 5 May, 2017. GoogleMaps
Paratypes. AMS R44772–73, Chillagoe Post Office, 14.9 km SE, NEQ ( 17°13'S, 144°33'E), 17 June, 1976 (also paratypes of L. storri ); AMS R113854, Chillagoe Post Office, 14.9 km SE, NEQ ( 17°16'S, 144°34'E), no collection date; QM J87270 View Materials , Almaden, NEQ ( 17°23'24"S, 144°39'39"E), 21 July GoogleMaps , 2007; QM J95787 View Materials , Savanna Way, S Almaden, NEQ ( 17°24'11"S, 144°38'54"E), 5 May GoogleMaps , 2017; QM J95792 View Materials , Savanna Way, S Almaden, NEQ ( 17°30'30"S, 144°36'54"E), 6 May GoogleMaps , 2017; QM J95806 View Materials , Savanna Way, S Almaden, NEQ ( 17°24'10"S, 144°38'55"E), 5 May, 2017 GoogleMaps .
Diagnosis. Distinguished from all other Lerista by the complete absence of a forelimb, hindlimb with a single clawed digit, interparietal distinct from the frontoparietals, prefrontals absent, usually only 2 supraciliaries and anterior chin shields in broad contact.
Comparisons. Lerista parameles sp. nov. is most closely related to L. ameles . It can be distinguished from this species by a hindlimb with a single, clawed digit ( vs. limbs entirely absent) and the number of supraciliaries (usually two vs. four). The species would key to L. storri following Cogger (2014); however it can be distinguished from this species by the longer hindlimbs (>3.4% SVL vs. <3.2% SVL) with a distinct, clawed digit ( vs. monostylar, claw absent), usually only two supraciliaries ( vs. three or four), usually two infralabials contacting the postmental ( vs. always only one) and anterior chin shields in broad contact ( vs. at most point contact). Only four other species of Lerista have two supraciliaries ( Lerista greeri Storr, 1982 , L. onsloviana Storr, 1984 , L. praefrontalis Greer, 1986 and L. stylis (Mitchell, 1955)) and these species also have no forelimb. However, all have three supraoculars ( vs. two), the supraciliaries in contact with each other ( vs. separated) and are geographically distant, occuring in WA or the Northern Territory (NT). One specimen of L. parameles sp. nov. had three supraciliaries, a condition shared with five other species ( Lerista desertorum ( Sternfeld, 1919) , L. edwardsae Storr, 1982 , L. elegans ( Gray, 1845) , L. lineata Bell, 1833 and L. puncticauda Storr, 1991 ). However, these species all have a forelimb with one or more clawed digits ( vs. forelimb entirely absent).
Description of holotype. SVL = 71 mm; HL = 5.2 mm, 7.3% SVL; HW = 3.4 mm, 65% HL; SE = 1.4 mm, 26% HL; eyelid free (not fused into a spectacle); EE = 2.6 mm, 49% HL; RL = 0.83 mm, 16% HL; NL = 1.0 mm, 19% HL; IN = 1.1 mm, 21% HL; EN = 1.8 mm, 34% HL; RF = 1.4 mm, 27% HL; E = 0.70 mm, 13% HL; ear minute, smaller than the surrounding scales; MW = 4.0 mm, 5.6% SVL; forelimb absent; L2 = 2.8 mm, 4.0% SVL; TL = 11 mm (broken). Hindlimb with a single clawed digit.
Midbody scale rows 18; NC = 38%; NaL = 19%; FN = 51%; FW = 111%; IW = 90%; PL = 59%; MV = 61%; two supraoculars; two supraciliaries, first enlarged and second at posterior edge of eye below last supraocular; first supraciliary contacts preocular, loreal, frontonasal, frontal and first supraocular; frontal contacts interparietal, frontoparietal, first supraocular, first supraciliary and frontonasal; interparietal free (not fused to frontoparietals); single loreal; prefrontal absent; single preocular; single presubocular; five palpebrals; single postocular; single postsubocular; five supralabials; third supralabial bordering eye; single postsupralabial; five infralabials, two infralabials contacting postmental; four scales between last infralabial and ear; single pretemporal; temporal contacts fourth and fifth supralabials, postocular, pretemporal, second temporal and lower temporal; three rows of enlarged chin shields; primary chin shields in broad contact; secondary chin shields separated by one scale; tertiary chin shields separated by three scales; five enlarged nuchal scales; 103 paravertebrals; two enlarged preanals; L2B = 5; five subdigital lamellae under single digit; two supradigitals.
Variation. Sample size is seven unless otherwise noted: SVL = 44–71 mm (63 + 9 mm); HL = 7.2–9.4% SVL (8.0 + 0.7%); HW = 22–72% HL (56 + 6%); SE = 23–34% HL (26 + 4%); EE = 43–59% HL (52 + 5%); RL = 14– 19 % HL (17 + 1%); NL = 19–23% HL (20 + 2%); IN = 19–25% HL (21 + 2%); EN = 28–38% HL (32 + 3%); RF = 25–30 % HL (28 + 2%); E = 12–17% HL (14 + 1%); MW = 5–7% SVL (6 + 1%); L2 = 3.4–4.7% SVL (3.9 + 0.5%); TL = 103% SVL (single original tail, QM J95792 View Materials , all others regrown).
Midbody scale rows 18–20 (mode = 18); NC = 36–52% (42 + 5%); NaL = 16–29% (20 + 5%); FN = 41–67% (54 + 8%); FW = 104–116% (109 + 5%); IW = 80–111% (90 + 10%); PL = 52–68% (59 + 5%); MV = 56–86% (74 + 12%); usually two supraciliaries, first enlarged and second at posterior edge of eye below last supraocular, sometimes a third supraciliary present between the first and second supraoculars not in contact with the other supraciliaries (N = 2, one side only); 3–6 palpebrals (mode = 4); usually first two infralabials contacting postmental (N = 6), sometimes one (N = 1); 4–5 scales between last infralabial and ear (mode = 5); temporal contacts fourth and fifth supralabials, postocular, pretemporal, second temporal and lower temporal (sometimes point contact with parietal, N = 3); 3–7 enlarged nuchal scales (mode = 4), 92–107 paravertebrals (mode = 105); L2B = 5–7 (mode = 6); 4 subdigital lamellae under single digit; 3 supradigitals; 94 subcaudals (N = 1).
Colouration in preservative ( Fig. 4 View FIGURE 4 ). The dorsal surfaces of the head, body and tail are pale beige to silvery grey. Body with a series of chocolate brown streaks that form broken to near continuous, longitudinal lines on back and flanks (scale rows one to six or seven), these extend along the length of the body and are continuous with those on the tail. The lateral stripes on the mid to lower flanks are usually broader than those on the dorsum. The lower flanks and ventral surface of the body are usually pale but may have a diffuse brown wash. The head shields bear obscure brown mottling. A dark zone is present from the secondary temporal, through the eye and extending forward to the ventral edge of the nasal. In AMS R44772 and AMS R44773, this extends as a dark wash encompassing the side of the face. The tail is strongly marked with longitudinal rows of dark streaks and heavily flecked on the ventral surface.
Colouration in life (as different from colouration in preservative, Fig. 11 View FIGURE 11 ). As for spirit specimens but the ground colour is more lustrous.
Etymology. The species epithet is formed from a combination of the Greek word para, meaning beside or near, and the species L. ameles , referring to the close relationship between this species and Lerista ameles .
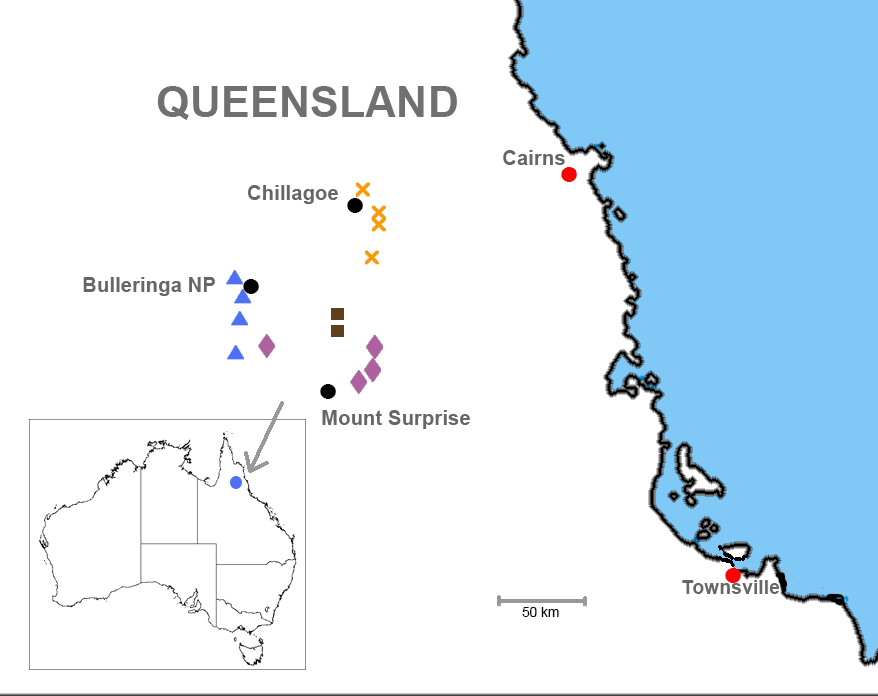
Distribution and habitat ( Figs. 1 View FIGURE 1 and 12 View FIGURE 12 ). Known only from the area surrounding Chillagoe in north-eastern Queensland with an EOO of approximately 256 km 2. AOO will be considerably less than this as areas of suitable habitat are small and patchily distributed within the EOO. This area is within the Lynd River catchment of the Mitchell River drainage system, in the Einasleigh Uplands bioregion of Queensland. Broadly speaking, the species inhabits loose, friable soil in open woodland. Queensland Regional Ecosystems in which this species was encountered are 912.7a and 9.12.27. These are defined as follows ( Queensland Government Environment and Science 2018):
9.12.7a: Woodland to open woodland of Eucalyptus cullenii +/- Corymbia erythrophloia +/- Erythrophleum chlorostachys +/- C. dallachiana . An open to mid-dense sub-canopy can occur and includes a variety of species. The shrub layer is absent to open and dominated by Denhamia cunninghamii, Alphitonia pomaderroides, Petalostigma spp., and Acacia spp. The ground layer is sparse to dense and dominated by Heteropogon contortus , H. triticeus , Themeda triandra and Sarga plumosum with a Xanthorrhoea sp. occurring in some areas. Occurs on rhyolite hills.
9.12.27: Low open woodland to woodland of Eucalyptus melanophloia or E. shirleyi +/- Corymbia erythrophloia +/- Acacia spp. A sub-canopy of taller shrub-layer species may be present. The shrub layer can be absent to scattered individuals of a wide mixture of species including Petalostigma banksia , Terminalia spp., Alphitonia pomaderroides, Gardenia vilhelmii, Denhamia cunninghamii and Grevillea glauca as well as canopy species. The ground layer is dense grassy and dominated by Schizachyrium spp., and Heteropogon contortus . Occurs on rolling hills and slopes with shallow soils on acid igneous geology, often with boulders to the surface.
General ecology. Specimens were found in loose soil under logs and other debris. As far as is known, it feeds on small arthropods.
Conservation status. Only three populations are known but more survey work may uncover new populations. It seems likely populations are naturally fragmented as the species is restricted to areas of suitable (sandy) soils and is probably unable to cross areas of unsuitable habitat. No known populations are in protected areas. They are subject to cattle grazing with its attendant soil impaction and ecological degradation, and invasive plant species altering soil structure. As the species has a known extent of occurrence well below 5,000 km 2 consisting of only three small populations, and is subjected to the direct threats of cattle grazing and weeds from which declines in the area of occupancy, habitat quality and number of individuals can be inferred, we suggest it qualifies as Endangered under the IUCN guidelines B1a, b (ii, iii, v) ( IUCN 2012).
| QM |
Queensland Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.