Merodon megavidus Vujić & Radenković, 2016
|
publication ID |
https://doi.org/ 10.5852/ejt.2016.237 |
|
persistent identifier |
https://treatment.plazi.org/id/03851D0F-3215-FFAC-7E8D-301EB23FFE2E |
|
treatment provided by |
Valdenar |
|
scientific name |
Merodon megavidus Vujić & Radenković |
| status |
sp. nov. |
Merodon megavidus Vujić & Radenković View in CoL sp. nov.
urn:lsid:zoobank.org:act:B2016D80-A7ED-4958-AE91-EE5E6A8C7C51
Figs 2 View Fig C–D, 3–5
Diagnosis
Medium- to large-sized species(13–18mm);black mesoscutum with four white microtrichose longitudinal stripes; tapering orange and black abdomen with white, transverse, microtrichose bands on tergites 2–4 (exceptionally without bands on tergite 2); tarsi reddish-orange dorsally; hind femur medium wide and slightly curved ( Fig. 3 View Fig C–D), with very short pile posteroventrally. Merodon megavidus Vujić & Radenković sp. nov. belongs to the avidus complex (male genitalia in all species identical in shape, as on Fig. 2 View Fig ). Merodon megavidus sp. nov. can be separated from the other members of the complex by larger size, golden body pile, bright orange colour of the pale parts of legs and extremely short pile on hind femur ( Fig. 3 View Fig C–D). These characteristics contrast with other species from the complex, which have yellow to grayish pale body pile and longer pile on the hind femur ( Fig. 3 View Fig A–B).
Etymology
The name megavidus refers to the large size (Greek word megas means “large”) and great similarity with Merodon avidus .
Type material
Holotype
GREECE: Ƌ, Lesvos , Petrounta, 26 Jul. 2015, leg. A. Vujić and S. Radenković ( FSUNS 10132 View Materials ). Paratypes
GREECE: Lesvos: 1 ♀, ( WML Misc 38). Agiassos: 1 Ƌ, 7 Jul. 2007, leg. M. Hull ( WML H723); 1 ♀, 8 Jun. 2003, leg. G. Ståhls ( FSUNS 04385); 1 ♀, 8 Jun. 2003, leg. G. Ståhls ( MZH); 1 Ƌ, 16 Jun. 2004, leg. M. Hull ( WML Hu-93); 3 ƋƋ, 19 Jun. 2003, leg. M. Hull ( WML Hu70, Hu-78, Hu-81); 1 Ƌ, 20 Apr. 2004, leg. M. Hull ( WML Hu-92); 4 ƋƋ, 20 Jun. 2004, leg. M. Hull ( WML Hu-77, Hu-94-96); 5 ƋƋ, 22 Jun. 2004, leg. M. Hull ( WML Hu-76, Hu-97-100); 2 ƋƋ, 22 Jun. 2004, leg. M. Hull ( FSUSN 04390, 04391); 2 ƋƋ, 22 Jun. 2009, leg. M. Hull ( WML H1432, H1433); 1 ♀, 23 Jun. 1999, leg. M. Hull ( FSUNS 04392); 3 ƋƋ, 23 Jun. 2003, leg. M. Hull ( WML Hu-69, Hu-79, Hu-80). Vatoussa: 16 ƋƋ, 10 ♀♀, 26 Jul. 2015, leg. A. Vujić and S. Radenković ( FSUNS 10133-10151, 10159, 10160, 10162, 10163, 10243, 10253, 10256); 1 Ƌ, 1–4 Jun. 2012, leg. Nakas ( FSUNS Ć94). Plomari: 2 ƋƋ, 14 Jul. 2004, ( FSUNS 02325, 03966); 1 Ƌ, 14. Jul. 2004, leg. H. Dahm (S532).
Description
Male ( Figs 2C View Fig , 3 View Fig , 4A View Fig , 5A View Fig )
HEAD ( Fig. 4A View Fig ). Antenna ( Fig. 4A View Fig ) orange, first flagellomere 1.8–2.0 times as long as wide, 2.0 times longer than pedicel, concave, apex acute; arista: second, third and basal part of fourth flagellomeres pale, fourth flagellomere dark brown in apical ½ and thickened basally, 1.4 times longer than first flagellomere; with short, dense microtrichia. Face and frons black, covered with long golden pile and silver, dense microtrichia. Oral margin shiny black, except for the lateral microtrichose areas ( Fig. 4A View Fig ). Vertical triangle isosceles, shiny black except in front of the anterior ocellus that has pale microtrichia, covered with long orange pile except for black pile on the ocellar triangle. Ocellar triangle equilateral. Eye contiguity about 12 ommatidia long. Vertical triangle: eye contiguity: ocellar triangle = 1.5: 0.7: 1. Eye pile dense, white. Occiput with orange pile, along the eye margin with dense white microtrichia and posteriorly with metallic, bluish-greenish lustre.
THORAX. Mesoscutum and scutellum black with bronze lustre, covered with relatively long, dense, erect golden pile. Side of mesoscutum above wing-base with a patch of black pile. Mesoscutum with two lateral and two submedian, longitudinal, white microtrichose stripes. Proepimeron, posterior anepisternum, anteroventral and posterodorsal part of katepisternum, anepimeron, metasternum and katatergite with long golden pile and grey-green microtrichia. Wing hyaline, with dense microtrichia; veins dark brown except for light brown C, Sc and R1. Calypter pale yellow. Haltere with light brown pedicel and yellow capitulum. Legs orange, except for the black basal ¾ of the front- and mid-femora. Pile on legs golden. Hind femur ( Fig. 3C View Fig ) moderately thickened and curved, about 3.6 times as long as deep. Pile on hind femur very short.
ABDOMEN ( Fig. 5A View Fig ). Dark with white microtrichose bands, tapering, 1.4 times longer than mesonotum (including scutellum). Tergites orange and reddish except for black tergite 1 and central parts of tergites 2–3 (and 4) ( Fig. 5A View Fig ); orange-reddish parts of variable size on tergite 3 and 4, laterally and along microtrichose bands. Tergites 2–4 each with a pair of white microtrichose marks (exceptionally absent only on tergite 2); tergites 3-4 with wide, oblique bands ( Fig. 5A View Fig ). Pile on tergites golden. Sternites translucent, orange to brown towards the tip of the abdomen, covered with long yellow pile.
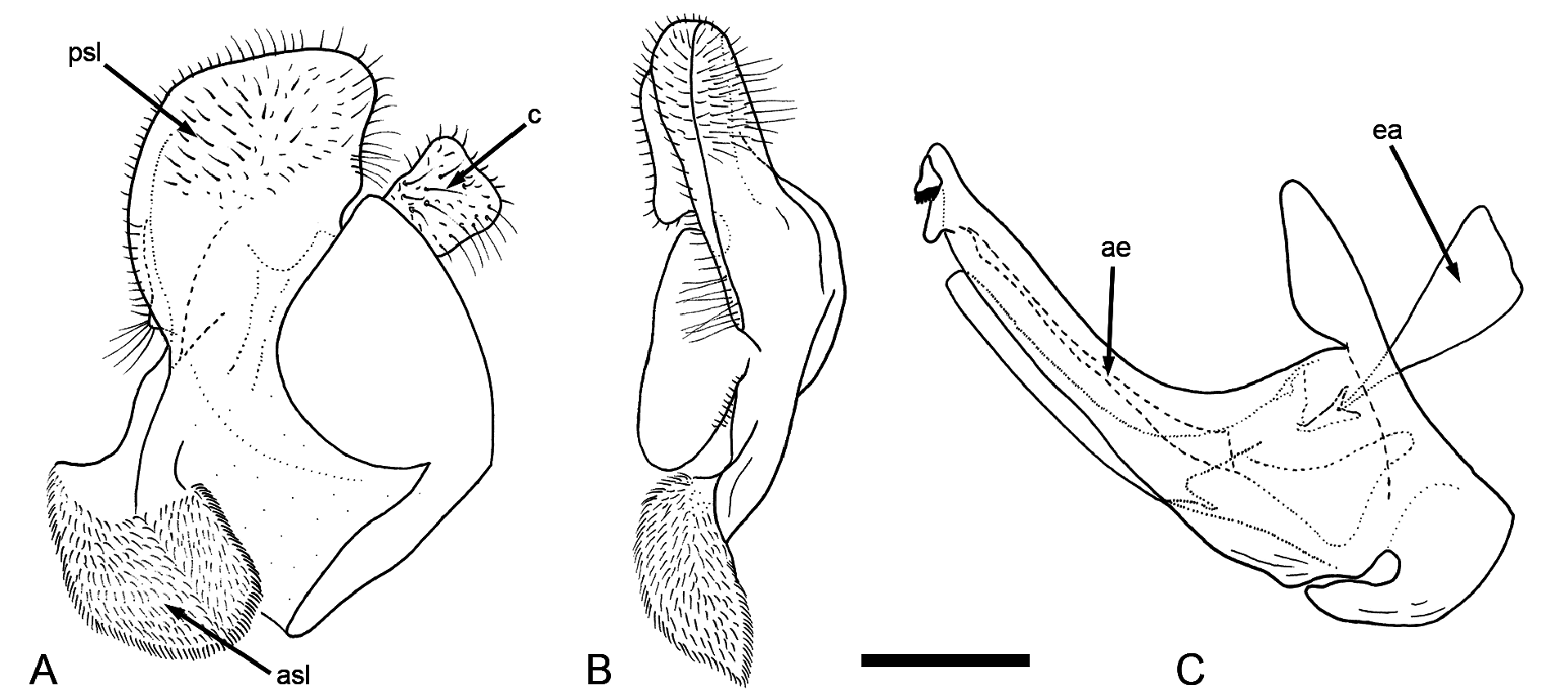
Male genitalia ( Fig. 2 View Fig ). Similar to all species of the M. avidus complex. Anterior lobe of surstylus broad and hairy ( Fig. 2 View Fig A–B); posterior lobe of surstylus ellipsoidal at ventral margin ( Fig. 2 View Fig A–B); cercus rectangular, without prominences ( Fig. 2A View Fig ). Hypandrium elongate and sickle–shaped, without lateral projections ( Fig. 2C View Fig ); lingula long ( Fig. 2C View Fig ).
Female ( Figs 2D View Fig , 4B View Fig , 5B View Fig )
Similar to the male except for typical sexual dimorphism and for the following characteristics: first flagellomere broader and longer; frons with two wide (about 0.34 width of frons) lateral silver microtrichose longitudinal stripes; frons in the widest part about 0.25 width of head; white microtrichose longitudinal stripes on mesoscutum more visible; broad stripe of black pile between wing bases; tergites predominately red except for tergite 1 and darkened parts of tergites 2–4 in some specimens ( Fig. 5B View Fig ); white, microtrichose, transverse bands on tergites 3–4 ( Fig. 5B View Fig ); tergites 2–3 with black pile on dark parts; white microtrichose bands solely with pale pile.
Remarks
This species was mentioned as Merodon sp. nova 2 in Ståhls et al. (2009) and Ricarte et al. (2012). Ståhls et al. (2009) assumed it to be a distinct species based on the different COI barcode sequences obtained from two specimens morphologically similar to M. avidus taken from Lesvos Island. In the key prepared as supporting material for Ståhls et al. (2009), M. avidus and M. sp. nova 2 key out together, without any morphological differences being described.
Distribution and habitat data
Lesvos Island ( Greece). Maquis shrubland.
Species delimitation
Molecular data
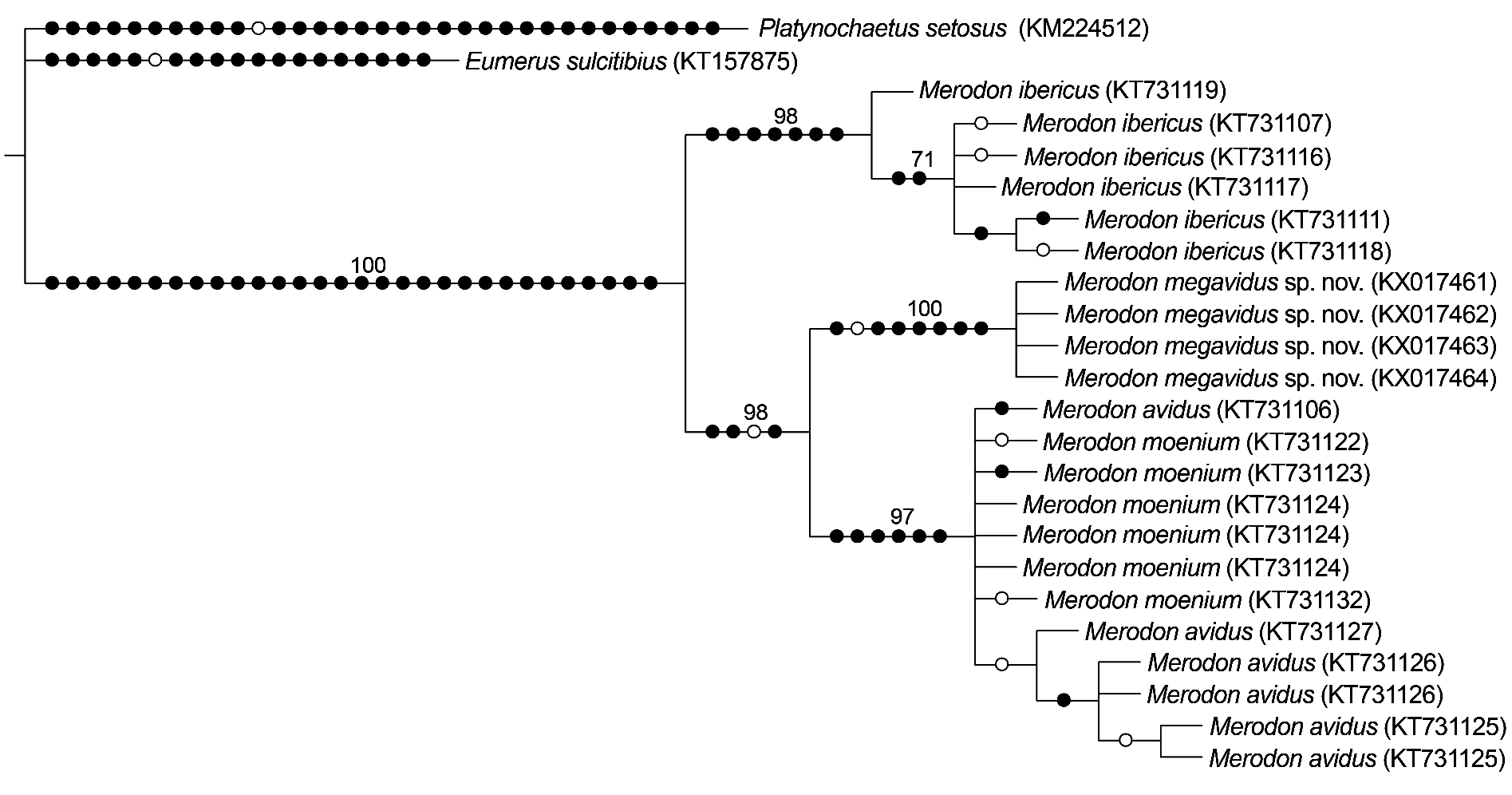
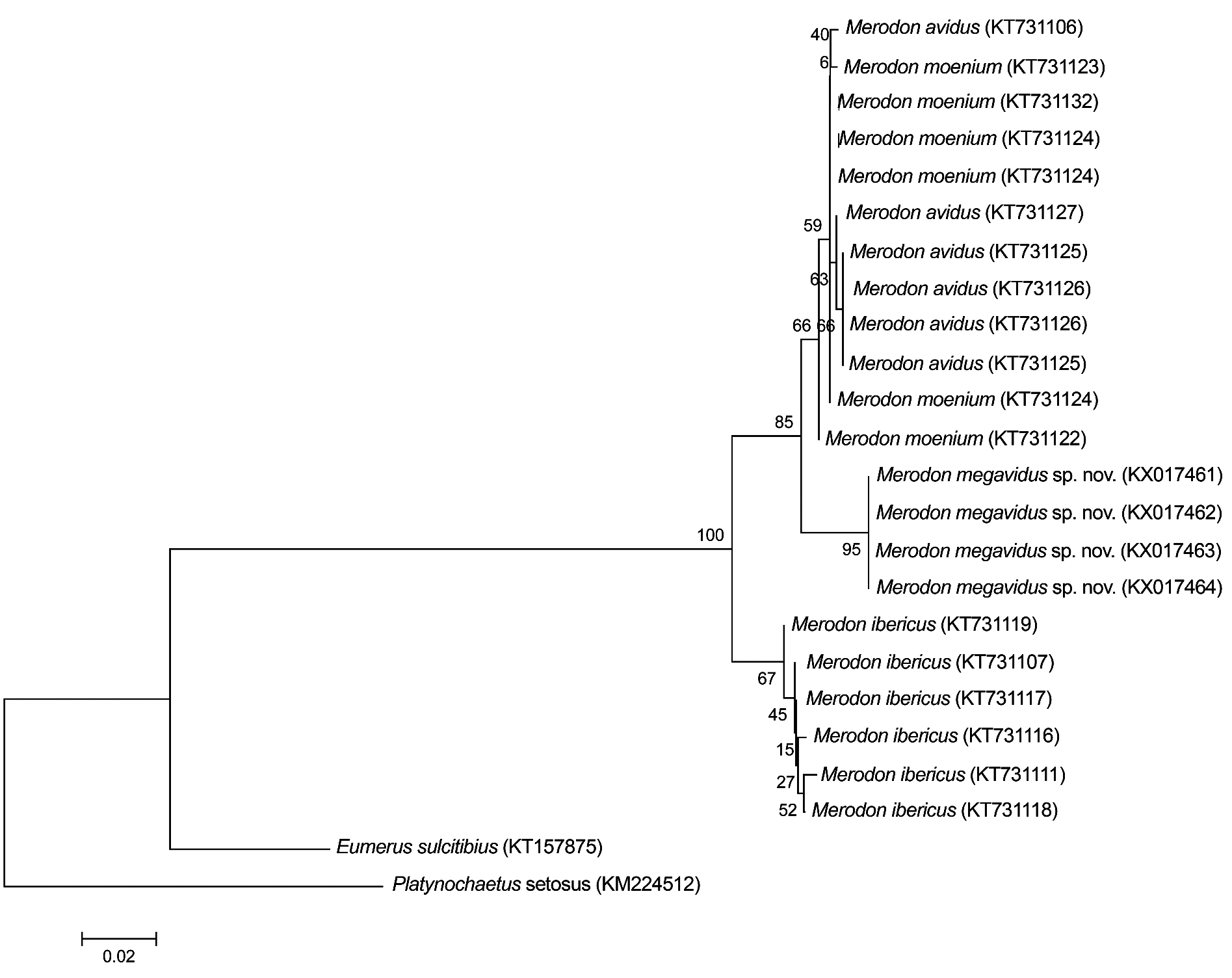
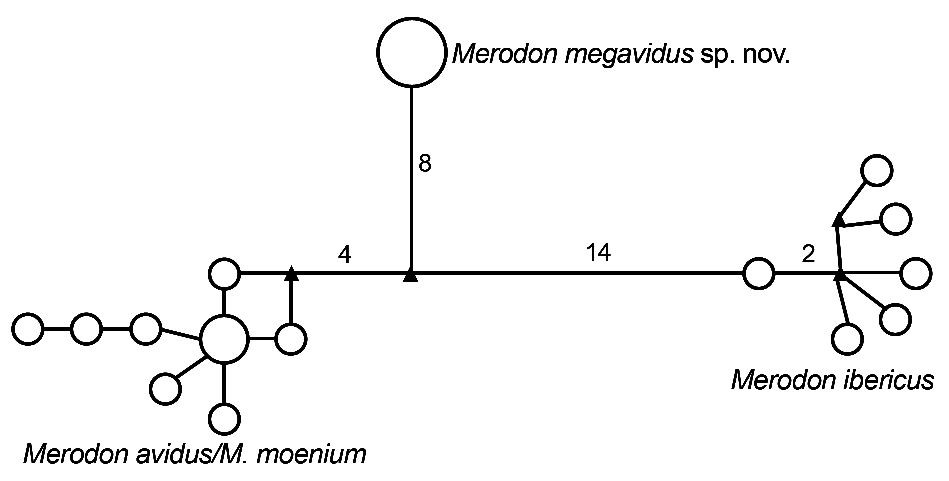
Merodon megavidus sp. nov. clearly differs from other members of M. avidus complex based on our barcoding fragment of COI. All conducted phylogenetic analyses resulted in similar tree topologies ( Figs 6–7 View Fig View Fig ; Appendix 3). Sequences from the Merodon avidus complex formed three separate clusters: one cluster represented M. ibericus , a second comprised all M. megavidus sp. nov. sequences with highly-significant bootstrap values (100), the third grouped sequences of M. avidus and M. moenium together. All four M. megavidus sp. nov. sequences were identical, defining one haplotype unique to the species. The number of mutational steps between M. megavidus sp. nov. and M. avidus is 14, with 13 and 22 mutational steps between M. megavidus sp. nov. and M. moenium and M. ibericus , respectively ( Fig. 8 View Fig ).
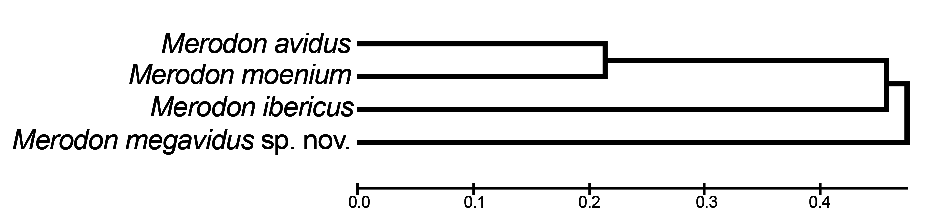
Our UPGMA tree based on genetic distances among species revealed genetic relationships among four taxa of the Merodon avidus complex that support and strengthen the branch positions in wing and surstylus phenograms described below (see Fig. 9 View Fig ).
Significant pairwise genetic divergence (ϕst value) was detected in each pairwise comparison between M. megavidus sp. nov. and each of the other species M. ibericus , M. avidus and M. moenium (0.420, 0.492 and 0.529, respectively). Sequence divergence (uncorrected p distance) of the COI gene was used to assess relative divergence times between the four Merodon taxa ( Table 1), indicating an initial separation of M. ibericus from the rest of the complex around 800 ky BP. Divergence between M. megavidus sp. nov. and M. avidus / M. moenium occurred around 500 ky BP. The most recent separation happened between M. avidus and M. moenium , around 87 ky BP.
Geometric morphometrics - wing shape analysis
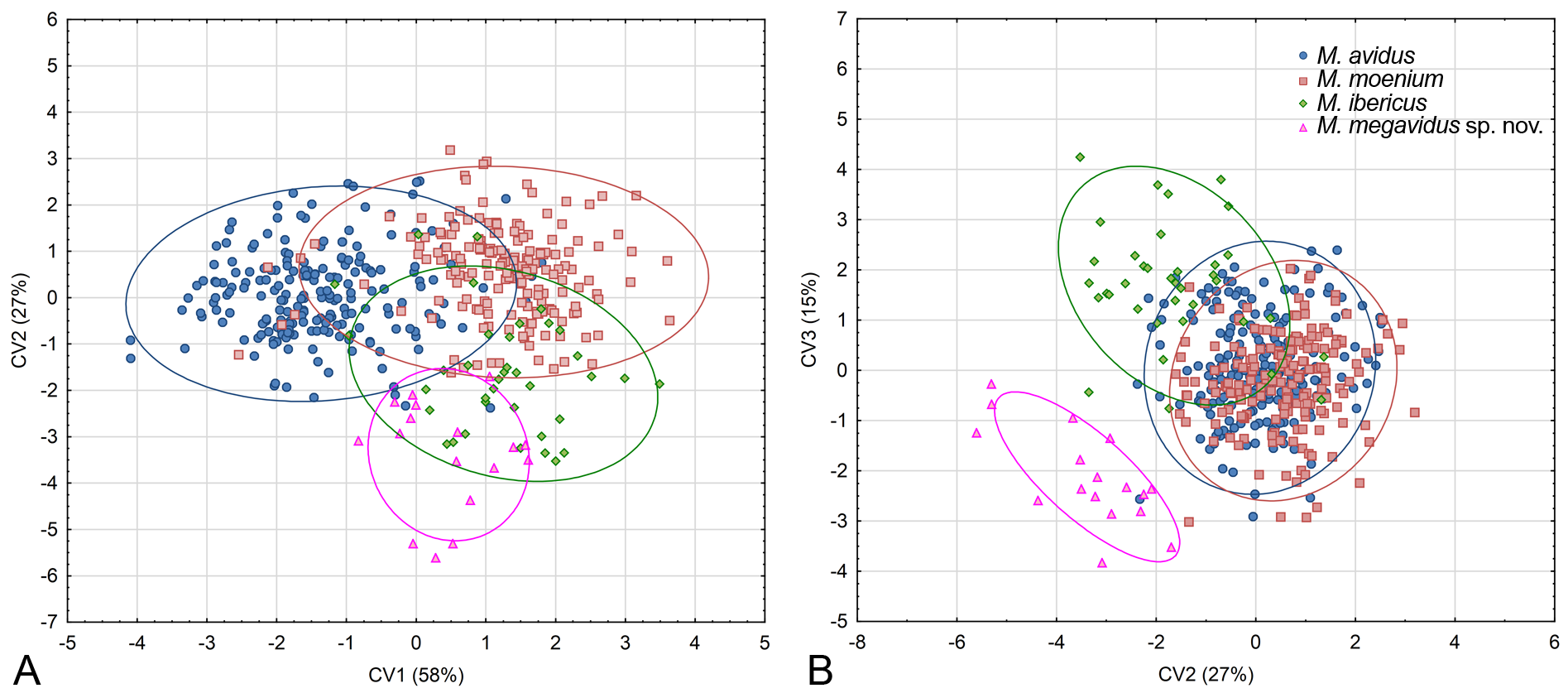
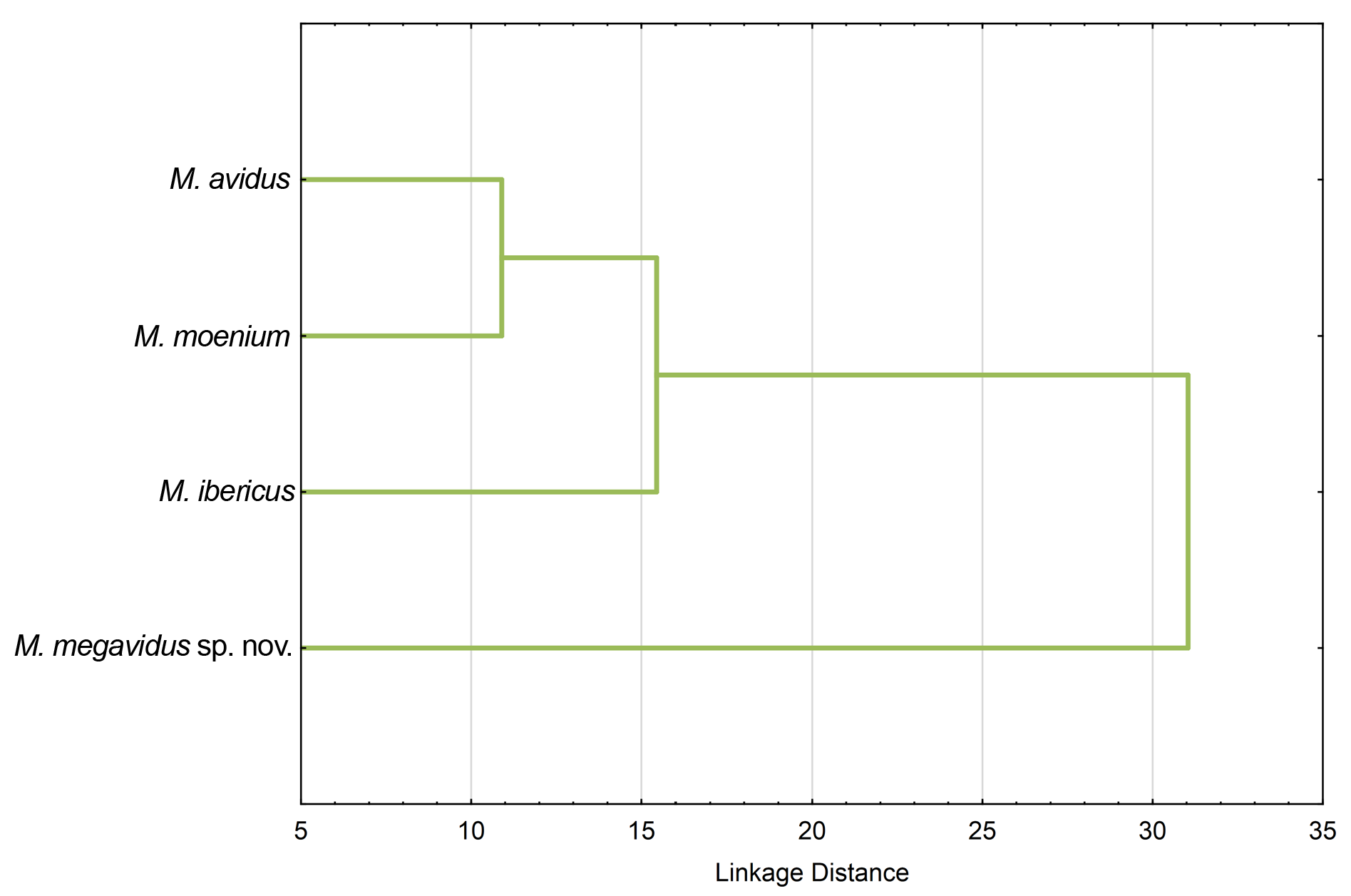
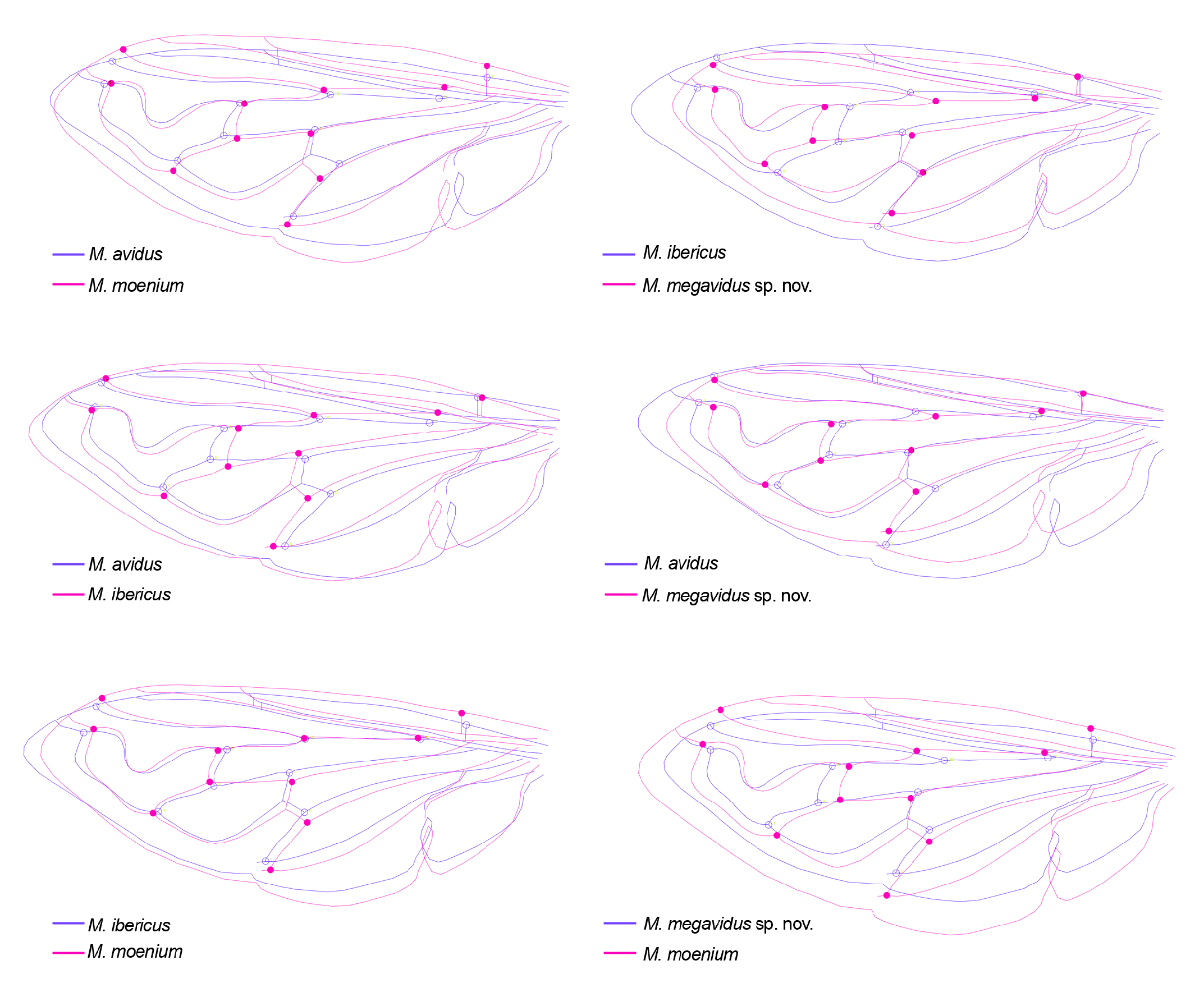
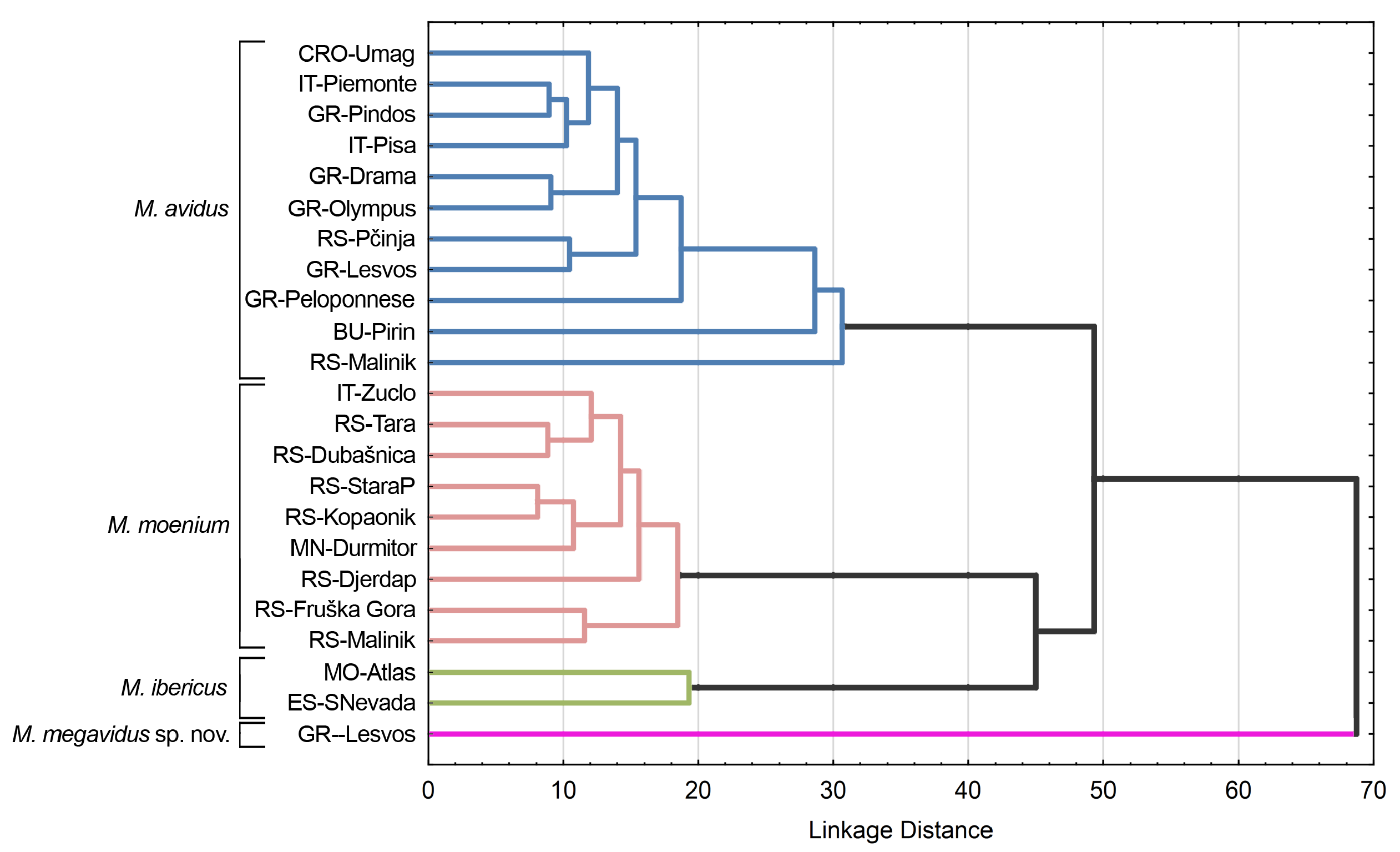
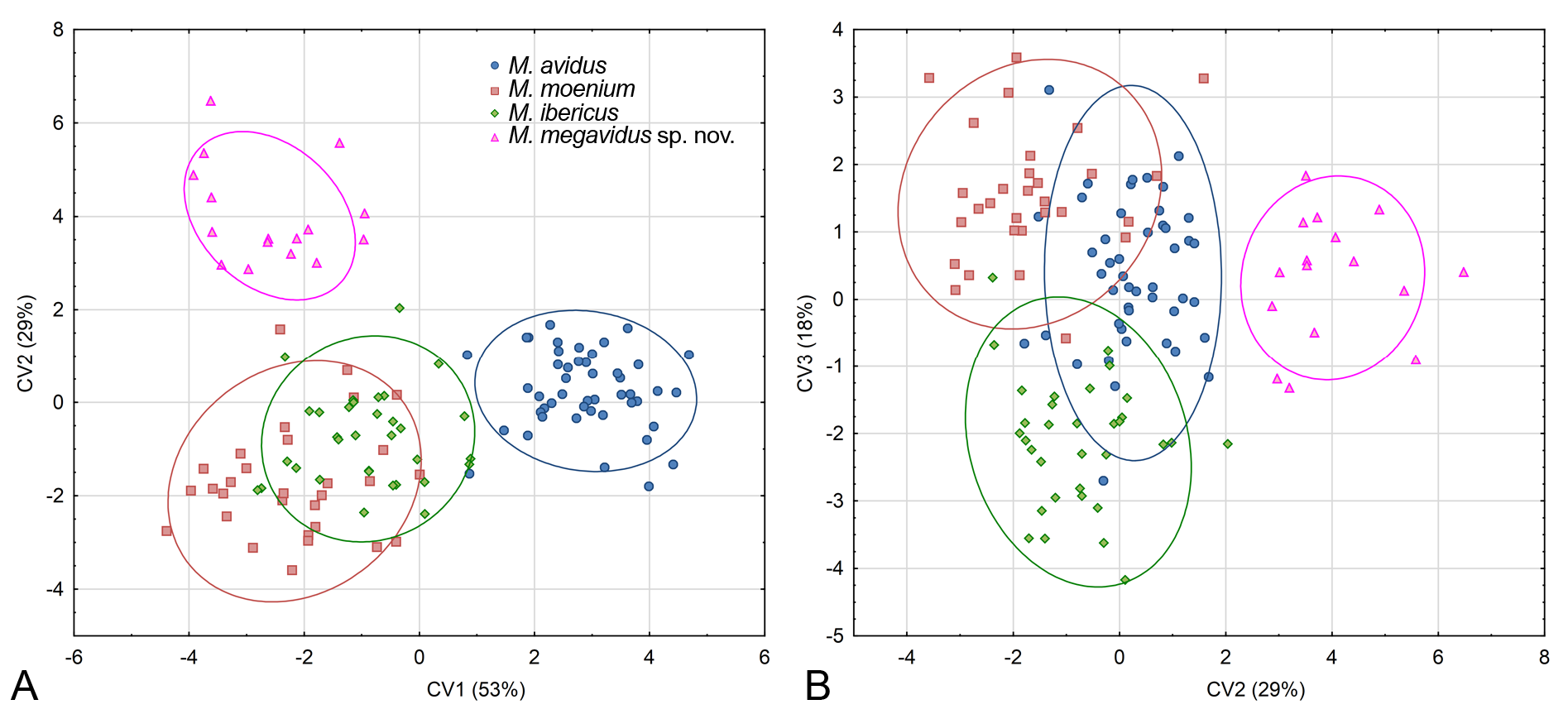
Principal component analysis conducted on 444 specimens of the M. avidus complex revealed six principal components (PC) that together explained 63.9% of total wing shape variability. A MANOVA with Fisher LSD post-hoc test showed that variability reflected shape changes among the investigated species in all six PCs (MANOVA: F 18,123 0 = 38.56; p <0.00000). Further, DA showed that all species differ highly significantly in wing shape (p <0.00000), and correctly classified species with an overall classification success of 90%. All specimens of M. megavidus sp. nov. were correctly classified, while the lowest classification success was for M. ibericus (78%). Specimens belonging to M. avidus were correctly classified with 89% and M. moenium with 93% certainty. Canonical analysis produced three canonical axes (CV) related to wing shape differences ( Fig. 10 View Fig ). CV1 separated M. avidus from M. moenium with 58% of total variation (CV1: Wilks’ Lambda = 0.145440; χ2 = 832.8937; p <0.00000), while CV2 separated M. avidus and M. moenium from M. megavidus sp. nov. and M. ibericus with 27% of total variation (CV2: Wilks’ Lambda = 0.388320; χ2 = 408.6395; p <0.00000) ( Fig. 10A View Fig ). Merodon moenium and M. ibericus were clearly separated according to CV3 (CV3: Wilks’ Lambda = 0.698600; χ2 = 154.9484; p <0.00000) ( Fig. 10B View Fig ). The phenogram constructed based on squared Mahalanobis distances clearly depicts that M. avidus and M. moenium have more similar wing shapes than M. ibericus and M. megavidus sp. nov. ( Fig. 11 View Fig ). Differences in wing shape among species are depicted in Figure 12 View Fig , but have been exaggerated five-fold to make them more visible.
Additionally, the phenogram constructed based on squared Mahalanobis distances of wing shape showed that all 26 analysed populations grouped according to species ( Fig. 13 View Fig ).
Geometric morphometrics - surstylus shape
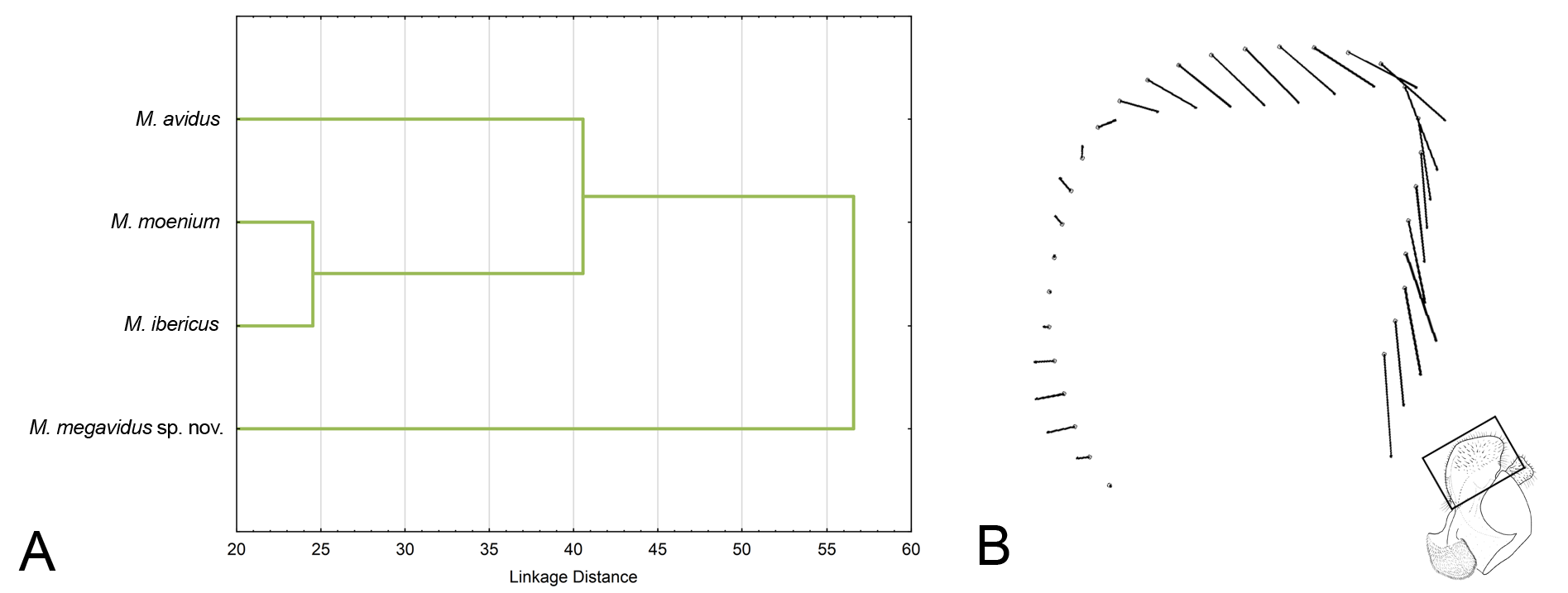
PCA of surstylus shape revealed seven PC, of which the first six were connected with shape differences among species (MANOVA: F 21,333 = 5.280113; p <0.00000). DA showed that all species differ highly significantly in surstylus shape (p <0.00000).All specimens of M. avidus and M. megavidus sp. nov. were correctly classified (100%), while only two specimens of M. ibericus and M. moenium were misclassified (97%). CVA found three CVs connected with shape change ( Fig. 14 View Fig ). CV1 clearly separated M. avidus from M. megavidus sp. nov., M. moenium and M. ibericus and represented 53% of total shape variability (CV1: Wilks’ Lambda = 0.013392; χ2 = 405.4285; p <0.00000) ( Fig. 14A View Fig ). The second canonical axis clearly separated M. megavidus sp. nov. from M. moenium and M. ibericus and was responsible for 25% of the variability (CV2: Wilks’ Lambda = 0.086702; χ2 = 229.8565; p <0.00000) ( Fig. 14A View Fig ). Merodon moenium and M. ibericus were separated by CV3, with 18% of total shape variability (CV3: Wilks’ Lambda = 0.346752; χ2 = 99.5597; p <0.000305) ( Fig. 14B View Fig ). According to the phenogram constructed based on squared Mahalanobis distances, M. megavidus sp. nov. has a more distinct surstylus shape, while the surstyli of M. moenium and M. ibericus are the most similar ( Fig. 15A View Fig ). The main shape differences among all three species lie in the posterior margin of the posterior part of the surstylus lobe ( Fig. 15A View Fig ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.