Nasiternella regia Riedel, 1914
|
publication ID |
https://doi.org/ 10.5281/zenodo.5739809 |
|
persistent identifier |
https://treatment.plazi.org/id/BE2287C9-FFEE-FFDE-C6B1-FBFADE4CD605 |
|
treatment provided by |
Marcus |
|
scientific name |
Nasiternella regia Riedel, 1914 |
| status |
|
Nasiternella regia Riedel, 1914 View in CoL
( Figs 1–7 View Fig View Figs 2–5 View Figs 6–7 )
Nasiternella regia Riedel, 1914: 150 View in CoL (description), Fig. 5 View Figs 2–5 (wings).
Nasiternella regia: LACKSCHEWITZ (1940) View in CoL :109 (faun.records); SAVCHENKO (1986):136 (redescription, based on RIEDEL 1914); SAVCHENKO (1989): 33 (references, distribution); SAVCHENKO et al. (1992): 196 (Palaearctic catalogue); OOSTERBROEK (2012) (World catalogue).
New type locality. Slovakia, Diviacka Nová Ves (district Prievidza), oak forest northeast of the village, 320 m a.s.l., 48°45′25.1″N, 18°30′43.5″E.
Type material examined. NEOTYPE: J (present designation), “ Slovakia 1.10.2011 / Diviacka Nová Ves (distr. / Prievidza), oak forest [7277] / J. Oboňa leg.”. Accordingly labelled as neotype (“ NEOTYPE / Nasiternella / regia Riedel / J. Starý & J. Oboňa 2012”) ( SNMC).The specimen has been pinned and the terminalia have been dissected and placed in a plastic tube with glycerine, pinned with the specimen.
Additional material examined (12 JJ 6 ♀♀): SLOVAKIA: the same locality as for neotype, 18.vi.2011 (ex larva, adult emerged 26.x.2011), 1 ♀ (pinned) ; 30.vii.2011 (ex larva, adult 26.x.2011), 1 ♀ (pinned) ; 27.ix.2011, 2 JJ (pinned, dried from ethanol); 1.x.2011, 4 JJ (pinned), 1 J 1 ♀ (in ethanol); 2.x.2011, 1 J (pinned); 9.iv.2012 (ex larva, adult 19.x.2012), 1 ♀ (pinned); 26.iv.2012 (ex larva, adult 14.x.2012), 1 J (pinned); 22.v.2012 (ex larva, adult 20.x.2012), 1 ♀ (pinned); 16.ix.2012 (ex pupa, adult 22.ix.2012), 1 J (pinned); 25.ix.2012, 1 J 1 ♀ (pinned), all J. Oboňa leg. (in JSOC, TUZS) . ALBANIA: Babia, 14.ix.1917, 1 J, Karny leg. ( NHMW) [not “20.xi.” as listed by LACKSCHEWITZ (1940)]. Years ago J. S. had examined the then single existing male of N. regia from the NHMW and made a sketch of the terminalia based on a (badly distorted) mount in Canada balsam between celluloid slides, probably prepared by Lackschewitz .
Diagnosis. Very large species. Body colouration yellowish brown. Prescutum with four dark brown stripes. Wing long, broad, intensively tinged with yellowish brown. Male terminalia with gonocoxite short, very broad at base, with powerful apical lobe; gonostylus bipartite, positioned at about midlength of gonocoxite. Female terminalia with cercus moderately upturned, obtuse at tip; spermathecae three, nearly spherical, small, and pale. Body length 15.3–22.6 mm, wing length 20.0–25.7 mm.
Redescription. Male. Head yellowish brown. Antenna short, 15-segmented, sometimes appearing 14-segmented, reaching slightly beyond anterior margin of prescutum. Basal segments yellowish brown, flagellomeres a little darkened distally. Flagellomere 1 subequal in length to scape, flagellomeres 2–10 shorter, short-ovoid, slightly decreasing in length and breadth towards apex of antenna, flagellomere 12 longer, cylindrical, terminal flagellomere somewhat conical, shorter than penultimate. Verticils on flagellomeres short, at most subequal in length to their respective segments, pubescence very short, barely evident. Palpus short, last palpomere about twice length of penultimate.
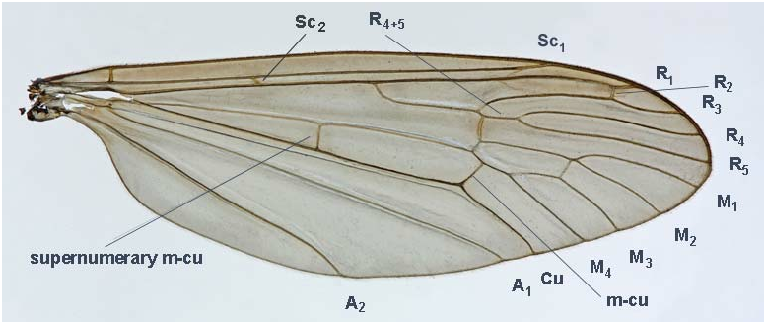
Thorax yellowish brown, with four dark brown prescutal stripes, inner ones incompletely separated from each other. Wing long, very broad, breadth about one-third its length, intensively tinged with yellowish brown, slightly more so along anterior margin. Veins pale, some cross-veins, especially r-m, slightly darker, with barely evident darkening on wing membrane. Wing generally delicate, veins weak, wing membrane distinctly wrinkled. Wing venation ( Fig. 1 View Fig ): Sc 2 more or less oblique, positioned at about two-thirds length of R. Rs arcuated at origin, without any spur. R 4 and R 5 four times (or more) as long as R 4+5. Cross-vein r-m connecting R 4+5 and M 1+2. Discal cell closed, distal section of M 1+2 (beyond discal cell) very short, sometimes cell M 1 almost sessile. Cross-vein m-cu at about one-third length of M 3+4 (lower side of discal cell). Supernumerary cross-vein in cell M distinctly before origin of Rs. Distance between of A 2 and A 1 at wing margin about six times or more that of A 1 and Cu. Halter yellowish brown. Legs yellowish brown, femora and tibiae with darker apices, distal tarsal segments darker. Metatarsus of fore leg subequal in length to tibia. Tarsal claws simple, lacking teeth along inner margin, slightly less than half length of last tarsal segment.
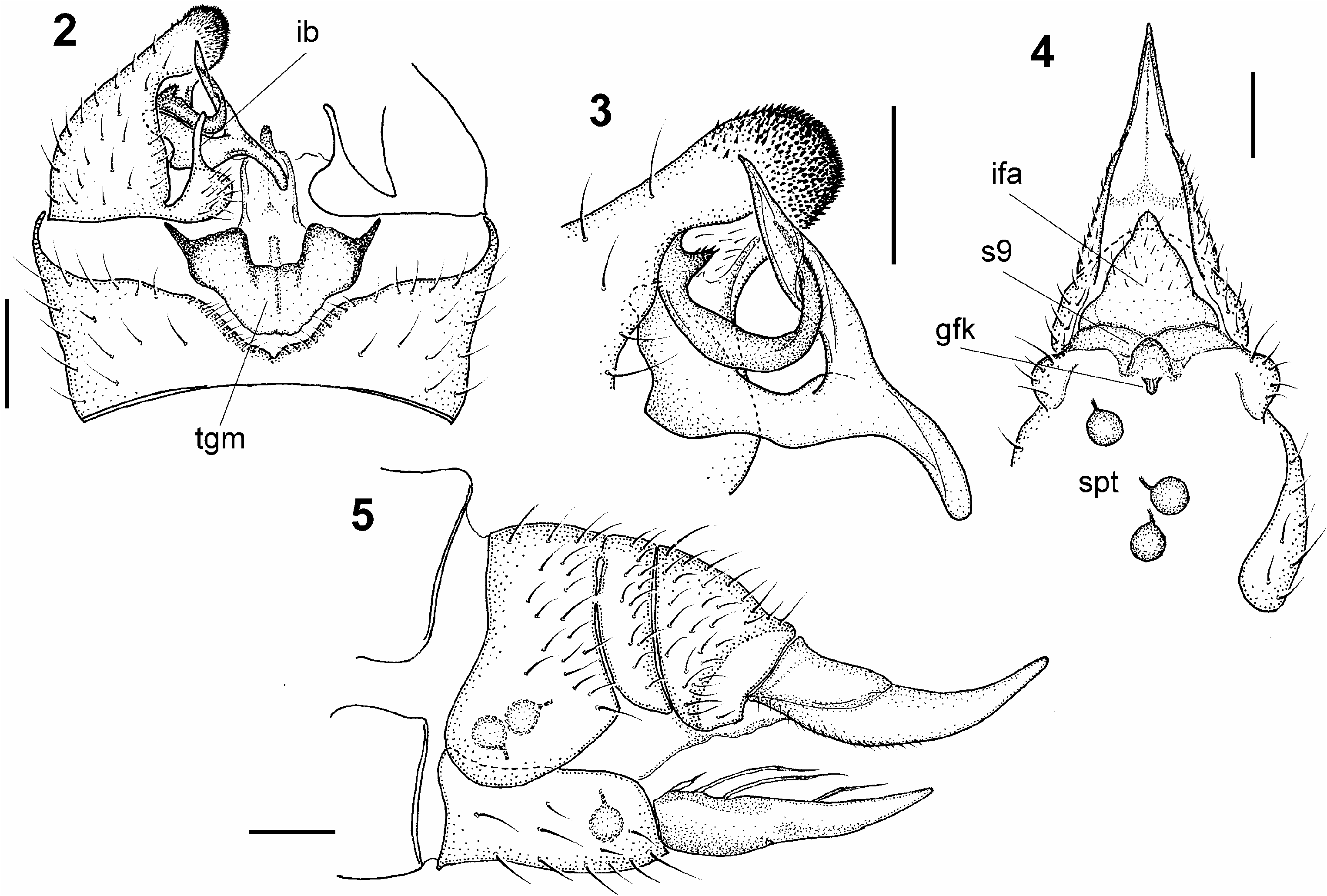
Abdomen yellowish brown, distal tergites incompletely dark brown medially, tracing out longitudinal stripe. All tergites very slightly darkened laterally. Male terminalia ( Figs 2–3 View Figs 2–5 ) yellowish brown. Segment 9 (basal ring) dorsally: short, broad, transverse, with median emargination at posterior margin; edge of emargination slightly darkened, with fringe of fine setae; ventrally: about twice as long as dorsally, thus gonocoxite connected to segment 9 at almost right angle. Gonocoxite generally short, very broad, inflated proximally, in distal third tapered into massive, broadly rounded apical lobe, at apex densely covered with black spinules. Gonostylus bipartite from base, arising roughly at midlength of gonocoxite. More proximal part of gonostylus, slightly more ventral in position, smooth, generally sinuous, obtuse at apex, with long, retrorse, somewhat twisted arm at about two-thirds its length, subacute at tip, extending back above inner margin of apical lobe of gonocoxite. More distal and dorsal part of gonostylus somewhat darker, covered with microscopic hairs distally, recurved at about midlength, with acute tip touching retrorse arm of proximal part of gonostylus, more or less rolled up in more proximal part. Small hump tipped with a few spinules present at outer base of distal part of gonostylus. Interbase pale, slender rod, obtuse at apex, arising from basal inner wall of gonocoxite. Aedeagal complex pale and comparatively inconspicuous, as usual for Pediciidae , except for large, transverse, median, darkly pigmented plate (tegmen of EDWARDS 1938 and DIENSKE 1987), situated dorsally slightly proximally of bases of gonocoxites, shaped as in Fig. 3 View Figs 2–5 (tgm), and normally partly concealed by membranous proctiger (the latter removed in Fig. 3 View Figs 2–5 ).
Female. In general appearance resembling male. Female terminalia ( Figs 4–5 View Figs 2–5 ) with cercus moderately long, gently upturned, obtuse at tip. Infra-anal plate triangular, rounded at apex, sparsely covered with setae. Sternite 9 as in Fig. 4 View Figs 2–5 (s9), forming transverse blade with elevated crescent-shaped central structure. Presumed genital fork very small, close to sternite 9. Hypogynial valve with four slender processes at inner margin, each tipped with seta, their lengths and configuration as in Fig. 5 View Figs 2–5 . Spermathecae three, nearly spherical, small, and weakly sclerotized, with sclerotized parts of ducts about half diameter of spermatheca.
Larva and pupa. For descriptions see OBOŇA & STARÝ (2013).
Differential diagnosis. The family Pediciidae contains some very large taxa such as Pedicia (s. str.) Latreille, 1809 and Malaisemyia . Within the genus Nasiternella , however, N. regia is quite unique in its size, being more than twice as large as its congeners. Beside its size, N. regia is distinctive by colouration of the wings. These are plain but intensively tinged with yellowish brown, whereas the wings in the other Nasiternella species are either conspicuously spotted ( N. hyperborea , N. ignara , N. varinervis ) or crossbanded ( N. tjederi ), or reduced to brachypterous condition in both sexes ( N. grallator ) ( ALEXANDER 1916, 1919, 1934, 1950, 1962). The structure of the male terminalia in N. regia suggests a closer relationship only to N. tjederi ( India: Sikkim) based on the shape of the gonostylus which is bipartite in both species ( Figs 2–3 View Figs 2–5 , cf. ALEXANDER 1962: Fig. 47). Nasiternella regia seems not to be as closely related to its European congener, N. varinervis . It should be noted that none of the specimens examined here has any abnormalities in the wing venation, as had both wings as illustrated for the female holotype of N. regia ( RIEDEL 1914: Fig. 5 View Figs 2–5 ).
Comment on neotype designation. Nasiternella regia was described by RIEDEL (1914) from a single female collected at Brassó (= Braşov) in Romania in a paper titled “Neue und wenig bekannte Limnobiiden aus dem Ungarischen National-Museum” [= New and little-known Limnobiidae from the Hungarian National Museum]. In the description, the depository of the holotype was again indicated (“Ung. Nat.-Mus.”, RIEDEL 1914: 150). No type, or any other specimen of N. regia is preserved in the HNHM, where it was most probably destroyed by fire during the Soviet army invasion in 1956 (G. Lengyel, pers. comm.). To fix the identity of the species, a neotype is designated here.
Biology. The immatures of N. regia were found to develop in water-filled tree holes, dendrotelmata, mostly of oak trees ( OBOŇA & STARÝ 2013). Of 18 specimens listed here from Slovakia (see above) 12 were captured in nature and 6 were reared from larvae/pupae. Of the adults collected from the field, all but one were taken from within tree holes during the day in sunny weather. To learn something about night activities of adults, on September 25, 2012, we adopted equipment used by collectors of moths, viz. a white vertical sheet illuminated with a mercury-vapour lamp. No specimen of N. regia was attracted to this light, although weather conditions seemed to be suitable. While searching with an electric torch during that same night a male was found on a tree trunk a short distance from his hollow. Adults appear to be negatively phototropic, leaving their shelters perhaps at twilight or by night, or maybe just on cloudy days, but their activity is likely to be mostly limited to crawling up and down the trees, because, despite their large wings, they seem to be bad fliers. A specimen removed from the tree hole and released from the hand took a short descending flight and settled to rest on the trunk of a nearby tree. This behaviour as well as the late imaginal occurrence (late September to early October) may be reasons for the seeming rarity of N. regia (this paper).
Distribution. Albania, Austria ( LACKSCHEWITZ 1940), Romania ( RIEDEL 1914), Slovakia ( OBOŇA & STARÝ 2013, this paper).
Discussion. The original description of N. regia (cf. RIEDEL 1914) gave mostly external characters; the female terminalia were only inadequately mentioned. Both wings were illustrated ( RIEDEL 1914: Fig. 5 View Figs 2–5 ), aberrant in the wing venation in a different manner, and the abnormalities were referred to in detail. Based on the external characters other than the wing venation, however, it is clear beyond any doubt that our examined specimens belong to N. regia .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Nasiternella regia Riedel, 1914
| Starý, Jaroslav & Oboňa, Jozef 2013 |
Nasiternella regia:
| SAVCHENKO E. N. & OOSTERBROEK P. & STARY J. 1992: 196 |
| SAVCHENKO E. N. 1989: 33 |
| SAVCHENKO E. N. 1986: 136 |
| LACKSCHEWITZ P. 1940: 109 |
Nasiternella regia
| RIEDEL M. P. 1914: 150 |