Phasmatocoris papei, Gil-Santana, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4413.3.4 |
|
publication LSID |
lsid:zoobank.org:pub:D60D3A99-4731-46C2-A879-8C9A3FCA5F21 |
|
DOI |
https://doi.org/10.5281/zenodo.5998024 |
|
persistent identifier |
https://treatment.plazi.org/id/039C87A0-FFF8-9C02-7D9E-F994364FFC32 |
|
treatment provided by |
Plazi |
|
scientific name |
Phasmatocoris papei |
| status |
sp. nov. |
Phasmatocoris papei View in CoL sp. nov.
( Figs. 1–34 View FIGURES1–5 View FIGURES6–10 View FIGURES 11–14 View FIGURES 15–20 View FIGURES 21–24 View FIGURES 25–26 View FIGURES 27–31 View FIGURES 32–34 )
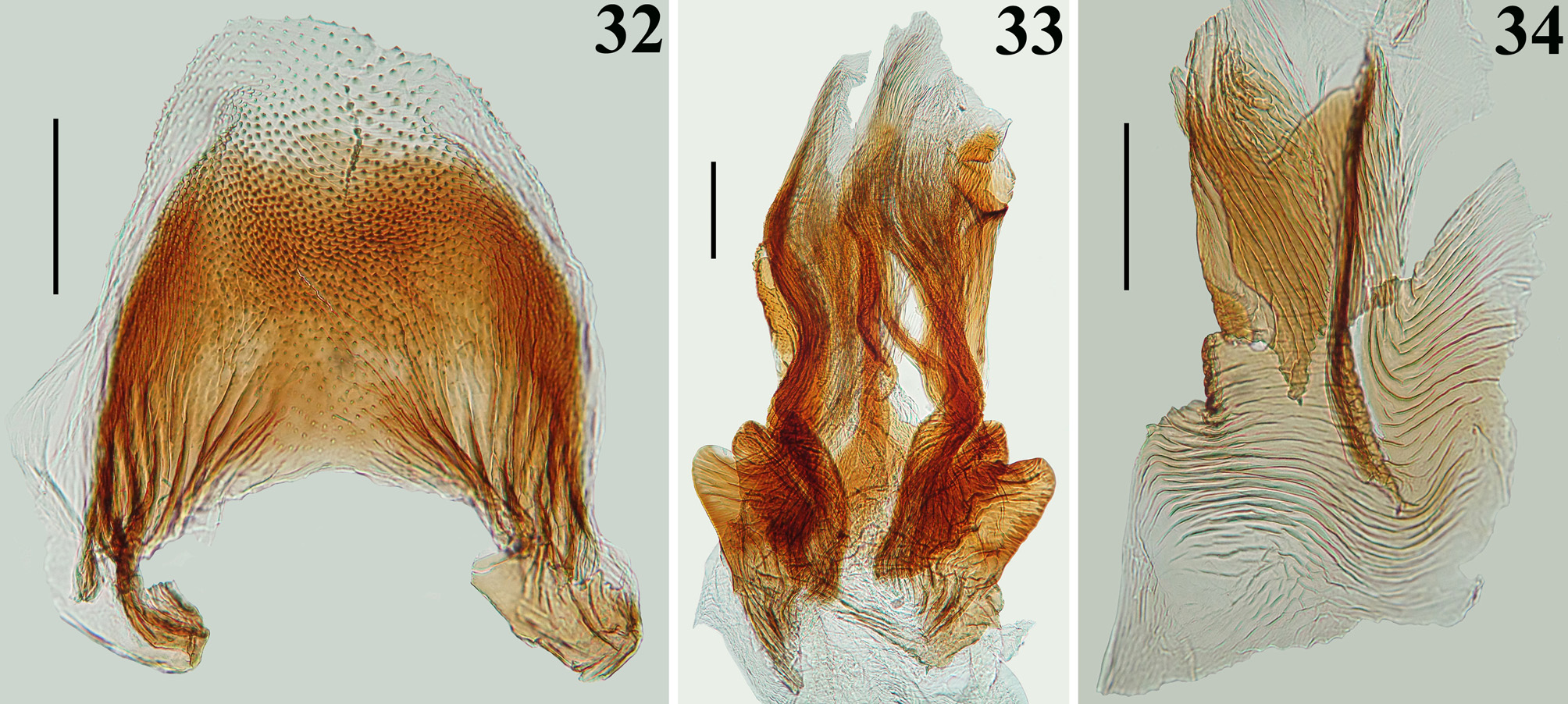
Diagnosis. The male most closely resembles that of Ph. moraballi Wygodzinsky, 1966 . The two species can be readily separated by the following characteristics: 1—longitudinal depression of hind lobe of pronotum almost imperceptible ( Ph. moraballi ) or wide, quite distinct ( Ph. papei sp. nov., Fig. 2 View FIGURES1–5 ); 2—subbasal and discal cells of forewing not completely separated ( Ph. moraballi ) or clearly separated by basal cell ( Ph. papei sp. nov., Figs. 12–14 View FIGURES 11–14 ); 3—parameres: a) shape of apical half of parameres subrectangular ( Ph. moraballi ) or suboval, with a pair of pointed processes at tip of inner surface ( Ph. papei sp. nov., Fig. 23 View FIGURES 21–24 ); b) their outer surface somewhat impressed on disc ( Ph. moraballi ) or not ( P. papei sp. nov., Fig. 24 View FIGURES 21–24 ); 4—phallothecal plate subrectangular ( Ph. moraballi ) or racket-shaped ( P. papei sp. nov., Figs. 28–29 View FIGURES 27–31 ); 5—endosoma processes clearly asymmetrical ( Ph. moraballi ), symmetrical or somewhat asymmetrical ( Ph. papei sp. nov., Figs. 32–34 View FIGURES 32–34 ).
Description. Male.
MEASUREMENTS: (holotype and paratype, in mm): total length: to tip of abdomen 12.5–13.7; to tip of forewings 12.1–13.3; head (excluding collum): length 1.5–1.6; length of anteocular portion 0.6; length of postocular portion 0.3–0.4; width across eyes 1.1; interocular distance between eyes 0.4; width of eye 0.4; length of eye: 0.5; lengths of antennal segments: I: 8.8–8.6; II: 8.0–7.8.; III: 1.1–1.0; IV: partially broken–1.9; lengths of labial segments: II [first visible]: 0.3; III: 0.5; IV: 0.9–1.0. Thorax: pronotum: fore lobe: length 1.8; maximum width 0.8; hind lobe: length 1.4; maximum width (at posterior margin) 1.3–1.4; length of forewing 8.2–8.4; length of hind wing 7.7–7.9. Fore legs: length of coxa 3.2–3.3; length of femur 4.6–4.7; length of tibia 2.3–2.2; length of tarsus 0.8; middle legs: length of femur 8.7–8.9; length of tibia 12.5–12.1; length of tarsus 0.5–absent; hind legs: length of femur 11.5; length of tibia 17.5; length of tarsus 0.5. Abdomen: length 7.5–7.8; maximum width 1.6.
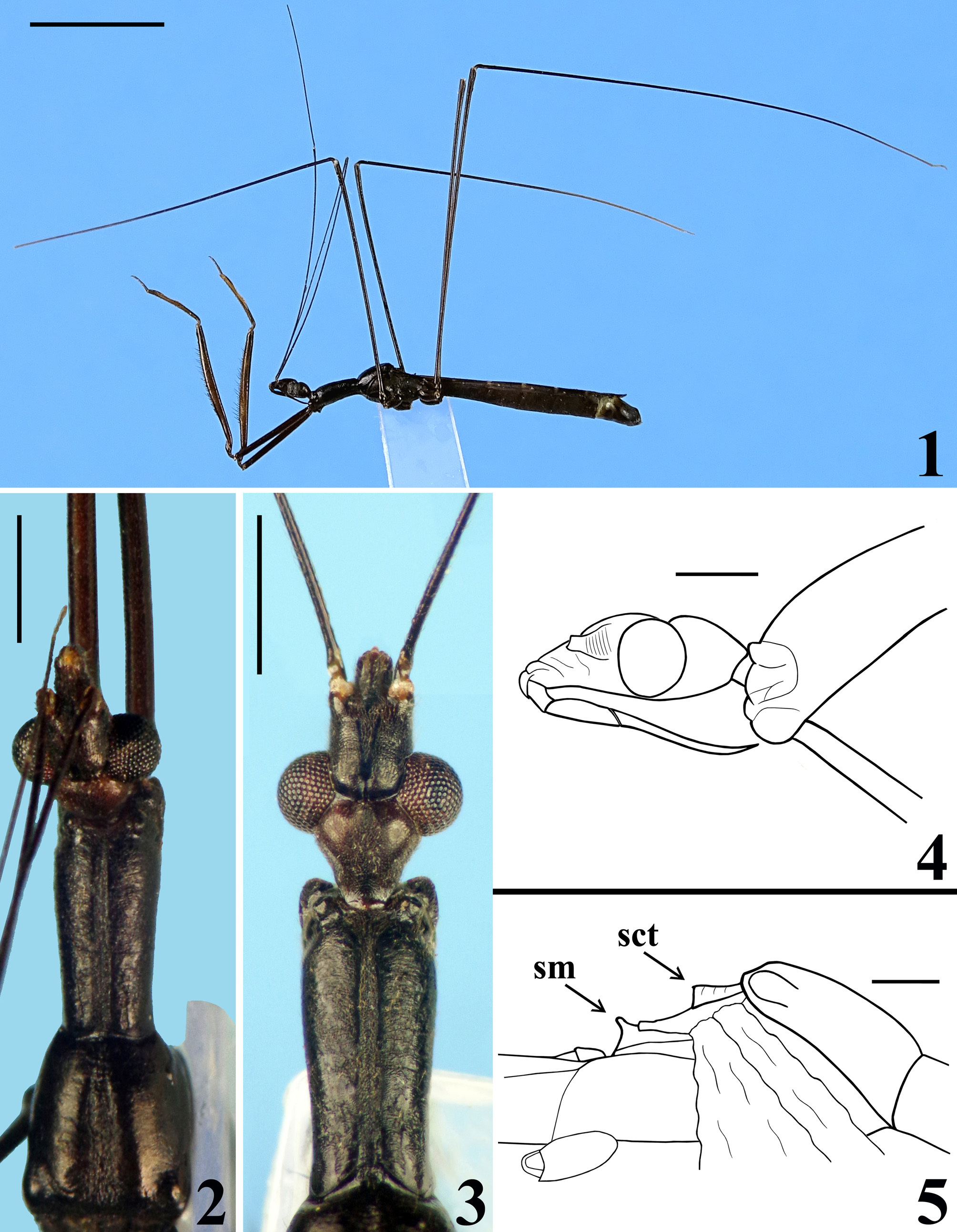
COLORATION: general coloration blackish ( Figs. 1–3 View FIGURES1–5 ); apices of antenniferous tubercles ( Fig. 3 View FIGURES1–5 ), labrum and tip of fourth antennal segment pale; labial segments III–IV brownish; fore tibiae, fore femora and fore tarsi brownish to brownish black; paratype with pairs of yellowish brown markings on inner and outer surfaces of fore femora, approximately at basal portion of mid and distal thirds of the segment; proximal marking of inner surface situated between isolated seta and basal seta of anteroventral series; only distal markings of fore femora evident in holotype, although faint; fore tibiae somewhat paler on basal portion of distal half; mid and hind tibiae with a small subbasal pale spot on inner surface and progressively paler towards apex, approximately at distal seventh of segment; mid and hind tarsi pale yellowish; forewings dark brown, veins enclosing basal, subbasal and discal cells somewhat lighter ( Fig. 11 View FIGURES 11–14 ); hind wings hyaline, veins somewhat and variably darkened; tergites III–VII brownish; connexivum darkened, connexival segments III–VII with small subrectangular basal pale markings ( Figs. 11 View FIGURES 11–14 , 16 View FIGURES 15–20 ); tongue-shaped prolongation of last tergite brownish with darkened margins ( Figs. 11 View FIGURES 11–14 , 16 View FIGURES 15–20 ); spiracles of the sternites III–VII and the area surrounding them whitish.
VESTITURE: body integument generally covered with a pubescence formed by very short slender adpressed setae and variably with scattered longer setae; paler or darker, similar to coloration of integument which they are inserted on. Head: mostly covered by a pubescence formed by adpressed short thin setae, with scattered longer setae on lateral region, clypeus, first two visible labial segments, and posteriorly to interocular sulcus. Labial segment IV almost glabrous, with scattered straight erect very short setae. Glabrous areas on head: transverse sulcus, a small median depressed area immediately anterior to it, and a pair of submedian slightly divergent thin bands running from midportion of transverse sulcus to a point near medial margin of antenniferous tubercles ( Fig. 3 View FIGURES1–5 ). Antenna covered with thin setae, longer and less numerous on first segment. Thorax: fore lobe of pronotum with sparse, short, adpressed, thin setae on dorsal and lateral portions, somewhat more numerous on anterior half; longer thin setae on lobe of acetabula and anterior projections of collar; hind lobe almost glabrous, with sparse somewhat curved setae; meso- and metapleura, pro-, meso- and metasterna covered by a somewhat sparse pubescence formed by short, thin, adpressed small setae; lateral portion of mesosternum glabrous. Wings glabrous. Legs covered by numerous short to somewhat longer thin setae. Armature of ventral surface of fore femora only with slender spine-like setae in two series, posteroventral and anteroventral, apically transformed into short teeth; anteroventral series interrupted at base, not connected to posteroventral series, one isolated seta basal to interruption ( Fig. 6 View FIGURES6–10 ); distance between this latter and first seta of series 0.4 mm; distance from base of fore femur to insertion of first spiniform seta of anteroventral series 1.9 / 2.1 mm, and from apex of fore trochanter 1.6 / 1.8 mm. Distance from base of fore femur to insertion of first spiniform seta in posteroventral series 1.3 / 1.4 mm, and from apex of fore trochanter, slightly longer than length of fore tarsus, 0.9 / 1.0 mm. Longer setae in posteroventral series, with length approximately as long as the value of maximum width of fore femur. Fore tibiae with a cluster of stiff obliquely inclined pale to golden setae on approximately distal half, dorsally, more numerous just basad to depressed area of this portion ( Figs. 7–9 View FIGURES6–10 ); numerous straight short setae close beside medial series of denticles ventrally ( Figs. 7–9 View FIGURES6–10 ); inner surface on approximately distal fourth, with dense short adpressed golden pubescence and a subapical comb ( Fig. 8 View FIGURES6–10 ); apex with a dense cluster of golden stiff setae ventrally ( Figs. 7–8, 10 View FIGURES6–10 ). Abdomen generally covered by a pubescence formed by short, thin, adpressed small setae.
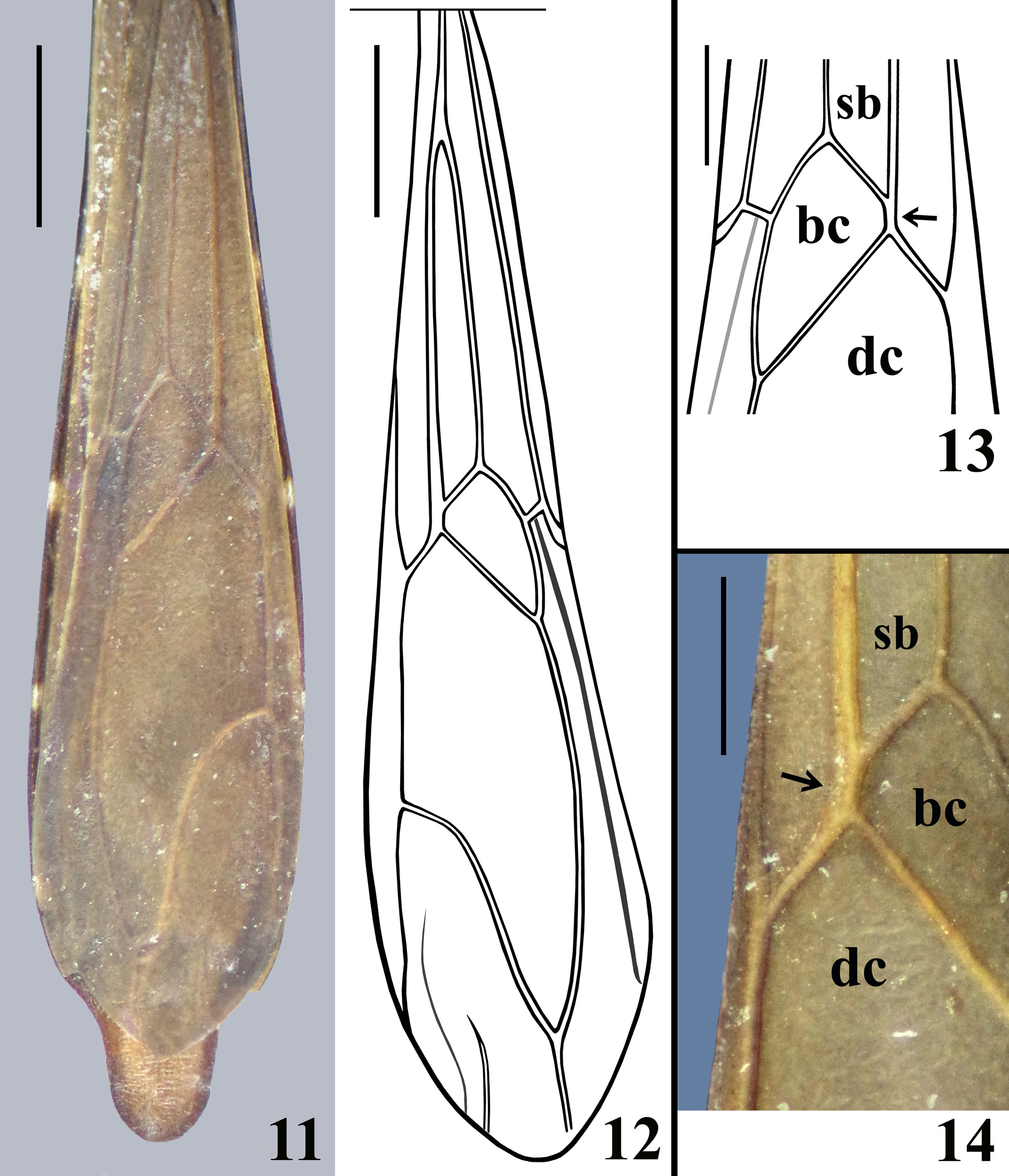
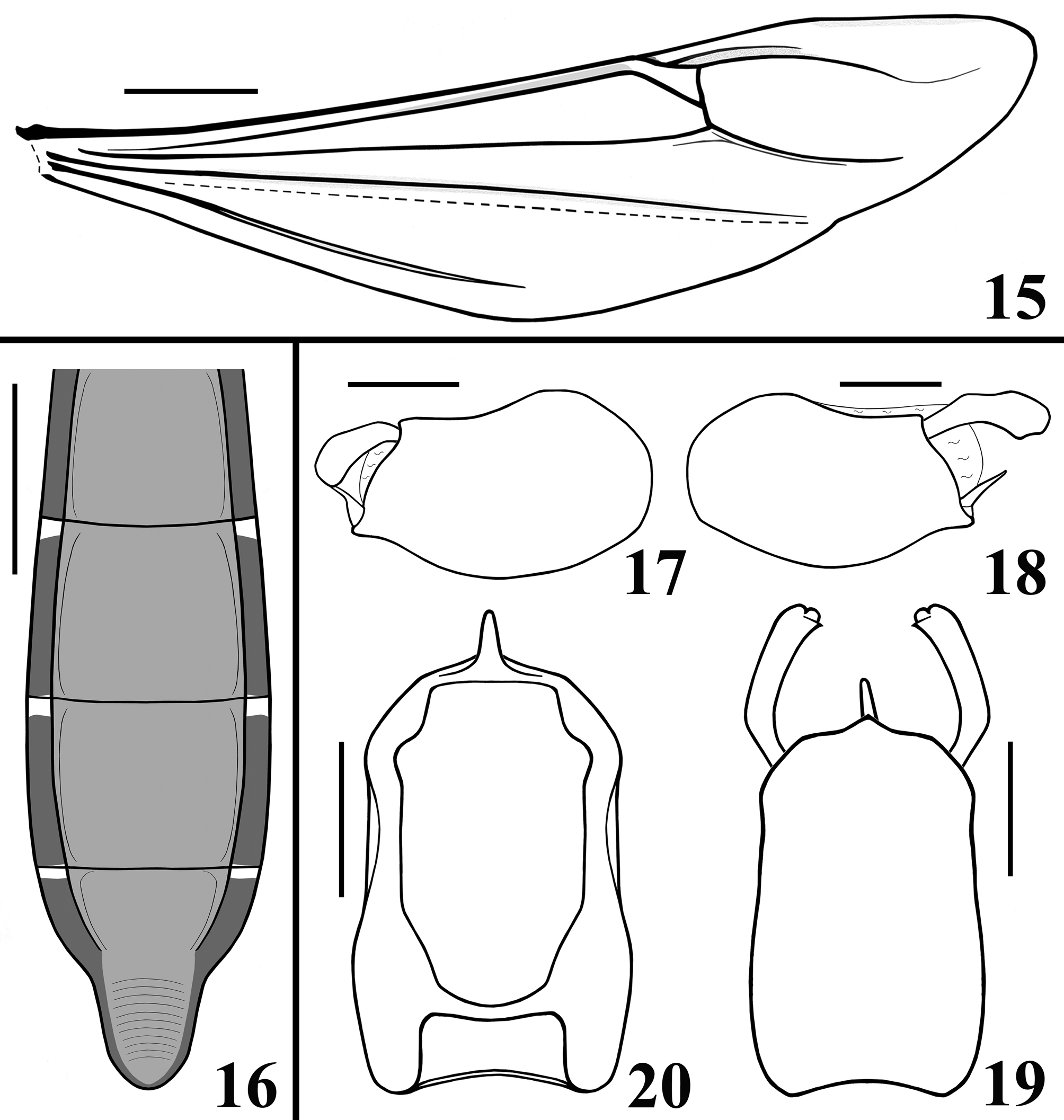
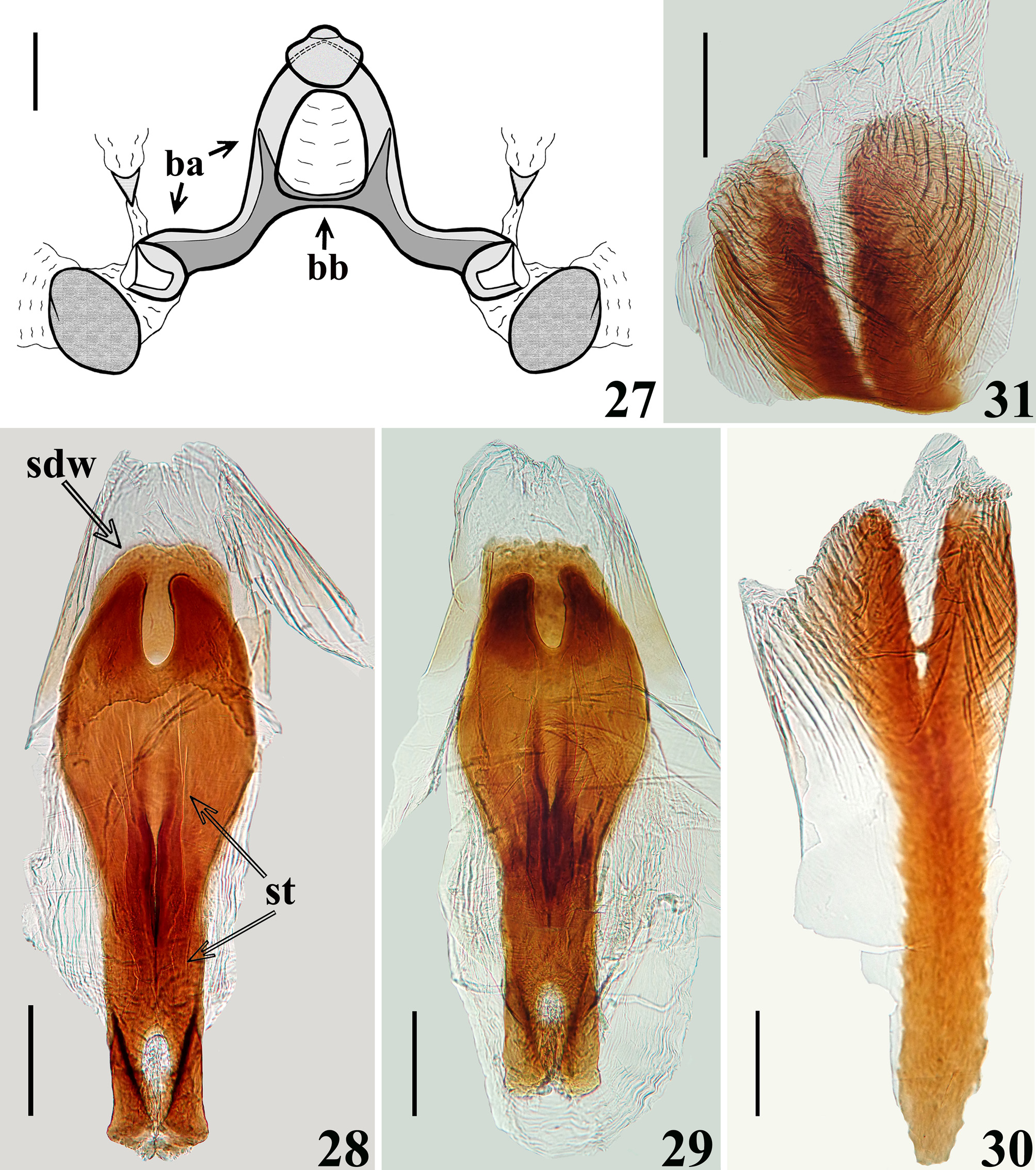
STRUCTURE: Integument moderately shiny. Head ( Figs. 3–4 View FIGURES1–5 ) elongated, approximately 1.4 times as long as wide across eyes; anteocular portion longer than postocular; transversal (interocular) sulcus deep, situated at level of middle third of eyes; a small median depressed area present immediately anteriad of it; distance from apex of antenniferous tubercle to anterior border of eye in lateral view (0.35 mm) shorter than length of eye (0.5 mm); eyes prominent, projecting laterally, subcircular in dorsal view, reaching dorsal outline of head at interocular sulcus and ventral margin of head. Antennal segments progressively thinner, very slender; segment IV with apex triangular. Labial segment III (second visible) thickest, apex approximately at level of anterior margin of eye; segment IV longest, ending at level of anterior extremity of stridulitrum ( Fig. 4 View FIGURES1–5 ). Thorax: fore lobe of pronotum approximately 1.3 times longer than hind lobe, sub-rectangular in dorsal view; anterior projections of collar rounded; integument rugose and transversely striated, dorsally and laterally; with a well-defined longitudinal medial shallow furrow, with rugose integument; transverse interlobar sulcus deep, curved ( Figs. 2–3 View FIGURES1–5 ). Hind lobe of pronotum shorter and larger than fore lobe; somewhat enlarged towards apical margin; integument very finely transversely striated, with a wide, distinct longitudinal shallow depression, enlarged towards apex, with rugose integument, posterior margin with rugose integument; humeral angles with elevated rounded tubercles ( Fig. 2 View FIGURES1–5 ). Lateral margins of stridulitrum conspicuous. Scutellum elevated, slightly prominent apically and with a small median rounded prominence at its tip ( Fig. 5 View FIGURES1–5 ). Metanotum with short but distinct spine ( Fig. 5 View FIGURES1–5 ). Fore coxae and fore femora elongated, latter somewhat broadened at middle; fore tibia approximately half as long as fore femora, dorsally depressed approximately at basal portion of distal half ( Figs. 7–9 View FIGURES6–10 ), ventrally with a single series of hook-like denticles ( Fig. 9 View FIGURES6–10 ), which are absent at basal portion and extreme apex; inner surface on distal quarter somewhat flattened ( Fig. 8 View FIGURES6–10 ). Tarsi three-segmented, slender ( Figs. 7–8, 10 View FIGURES6–10 ). Mid and hind femora and tibiae very long and slender ( Fig. 1 View FIGURES1–5 ). Forewings approaching apex of abdomen by approximately 0.4 mm ( Fig. 11 View FIGURES 11–14 ); subbasal cell longer than basal cell ( Figs. 11–12 View FIGURES 11–14 ); subbasal and discal cells clearly separated by basal cell ( Figs. 11–14 View FIGURES 11–14 ); shortest distance between apex of subbasal cell and base of discal cell somewhat longer in paratype than in holotype ( Figs. 13–14 View FIGURES 11–14 ). Hind wings reaching basal portion of tergite VII; venation as in Fig. 15 View FIGURES 15–20 . Abdomen: segment II narrow; segments III–V progressively slightly widened towards apex; segment VI slightly narrowed towards apex; segment VII strongly narrowed towards apex, dorsally with a tongueshaped prolongation posteriorly, somewhat wider at base and with a rounded posterior margin ( Figs. 11 View FIGURES 11–14 , 16 View FIGURES 15–20 ). Connexival segments simple, straight ( Fig. 16 View FIGURES 15–20 ). Sternites with a median longitudinal thin and shallow carina. Eight sternite with posterior margin slightly prolonged medially. Male genitalia ( Figs. 17–34 View FIGURES 15–20 View FIGURES 21–24 View FIGURES 25–26 View FIGURES 27–31 View FIGURES 32–34 ): pygophore subrectangular in dorsal view ( Fig. 20 View FIGURES 15–20 ); process of pygophore spine-like ( Figs. 17–22 View FIGURES 15–20 View FIGURES 21–24 ). Parameres symmetrical ( Figs. 19 View FIGURES 15–20 , 22 View FIGURES 21–24 ), curved, with apical half enlarged, suboval in shape ( Figs. 17–18 View FIGURES 15–20 , 22–24 View FIGURES 21–24 ); outer surface densely setose, not impressed on disc ( Fig. 24 View FIGURES 21–24 ); inner surface less setose, with a pair of pointed processes at its tip ( Fig. 23 View FIGURES 21–24 ). Phallus ( Figs. 25–26 View FIGURES 25–26 ): articulatory apparatus (art app) with basal arms (ba) shorter than phallosoma, divergent, connected by a short basal bridge (bb) ( Figs. 25–27 View FIGURES 25–26 View FIGURES 27–31 ); dorsal phallothecal plate (dp) ( Figs. 28–29 View FIGURES 27–31 ) racket-shaped, basal half with subparallel margins, somewhat narrowing towards mid portion; distal half suboval; narrowly to somewhat divided at basal portion and largely divided at apical portion; apical extremities rounded and somewhat asymmetrical; struts (st) separated at approximately apical third (paratype) ( Fig. 28 View FIGURES 27–31 ) to apical half (holotype) ( Fig. 29 View FIGURES 27–31 ). A short sclerotization of dorsal wall of phallosoma (sdw) ventrally and distally to the apical portion of dorsal phallothecal plate ( Figs. 28–29 View FIGURES 27–31 ). Paratype with a somewhat elongated sclerotization of ventral wall of phallosoma (svw) ( Figs. 26 View FIGURES 25–26 , 30 View FIGURES 27–31 ), which becomes divided in two slightly divergent branches approximately in the apical third (asvw), with lateral margins striated and apical margin continuous with dorsal membranous wall ( Fig. 30 View FIGURES 27–31 ); in holotype, only apical third (asvw) sclerotized ( Figs. 25 View FIGURES 25–26 ), forming a subsquared, somewhat larger and asymmetrical flat process, very weakly attached to the respective not sclerotized basal portion of ventral wall ( Figs. 25 View FIGURES 25–26 , 31 View FIGURES 27–31 ); this process positioned laterally in holotype ( Fig. 25 View FIGURES 25–26 ), possibly because its weak attachments to ventral wall. Endosoma wall smooth. Endosoma with three processes: 1—a somewhat large, flat, rounded, spiny, somewhat sclerotized dorsal process (dps) ( Figs. 25–26 View FIGURES 25–26 , 32 View FIGURES 32–34 ); 2—a large process (lps) with a sclerotized elongated core surrounded by filamentous and membranous layers, enlarged at base and somewhat narrowed and asymmetrical towards apical third, in which at one side only, a somewhat curved sclerotized band ( Figs. 25–26 View FIGURES 25–26 , 33 View FIGURES 32–34 ); 3—a ventral, flat, thin, somewhat enlarged, subrectangular, striated process (vps) ( Figs. 25–26 View FIGURES 25–26 , 34 View FIGURES 32–34 ).
Distribution. French Guiana.
Etymology. The new species is named in honor of Dr. Robert B. Pape (Department of Entomology, University of Arizona, Tucson, Arizona, USA) for his great contribution to entomology. Type material. FRENCH GUIANA, Bélizon, vii.2001, leg. H. Gaspard (male holotype, MNHN); same but i.2001 (1 male paratype, MNHN).
Comments. The inclusion of Ph. papei sp. nov. in Phasmatocoris is in accordance with the characteristics assigned to this genus by Wygodzinsky (1966), Forero (2004), Pape (2013) and Gil-Santana (2015).
In this matter, it is noteworthy to mention that Wygodzinsky (1966) argued that Phasmatocoris was distinguished from all other genera of Emesini by the considerably asymmetrical phallus. The asymmetry would often be perceptible in the phallosoma, especially in the shape of the basal plate extension and struts and the overlying dorsal sclerotization of the phallosoma wall. The greatest degree of asymmetry, however, was found in the endosoma, especially in its apical portion ( Wygodzinsky 1966).
However, the male genitalia of Ph. papei sp. nov. is basically symmetrical, with slight asymmetry in the apical extremities of dorsal phallothecal plate (dp) ( Figs. 28–29 View FIGURES 27–31 ), apical thirds of the larger process of the endosoma (lps) ( Fig. 33 View FIGURES 32–34 ) and sclerotization of the ventral wall of phallosoma (asvw) ( Figs. 30–31 View FIGURES 27–31 ), the latter more prominently in the holotype.
On the other hand, in other four species of Phasmatocoris, Gil-Santana (2015) found a considerable range in the degree of asymmetry in the phallosoma and processes of the endosoma, from quite asymmetrical to basically symmetrical or only slightly asymmetrical. Therefore, the slight asymmetry recorded in phallic structures of Ph. papei sp. nov. are in accordance with the observations of the latter author that there is a range in the degree of the asymmetry in the phallic structures among the species of Phasmatocoris .
The examined specimens of Ph. papei sp. nov. are very similar in general coloration, structure and vestiture. A minor variation in yellowish brown markings of fore femora was observed (paratype with two pairs of markings, while in the holotype only the distal markings of the fore femora are evident, although faint too). The shortest distance between the apex of the subbasal cell and the base of the discal cell is somewhat longer in the paratype ( Figs. 13– 14 View FIGURES 11–14 ).
However, an important variation was observed in the male genitalia, i.e., the absence (holotype) / presence (paratype) of the sclerotization of approximately basal two thirds of the ventral wall of phallosoma ( Figs. 25–26 View FIGURES 25–26 , 30–31 View FIGURES 27–31 ). This variation is noteworthy because the basal sclerotization of ventral wall can potentially be considered taxonomically relevant and also because the lack of it in the holotype apparently prompted the lateral positioning of the sclerotized enlarged apical third ( Fig. 25 View FIGURES 25–26 , asvw).
The latter, if recorded alone, could have been considered as another endosomal process and not as the apical sclerotization of the ventral wall, while its lateral positioning could be interpreted as asymmetry of the phallus. Another minor variation was also recorded in male genitalia, i.e., the struts were separated approximately at their apical thirds (paratype) ( Fig. 28 View FIGURES 27–31 ) or apical halves (holotype) ( Fig. 29 View FIGURES 27–31 ).
Because in the taxonomic practice it is common to dissect the male genitalia of a single specimen of each species only ( Lent & Jurberg 1985), the variability of these structures may remain unrecorded ( Gil-Santana et al. 2013). Therefore it is advisable to dissect more than one specimen of each species in order to ascertain the possible variability of the male genitalia.
Phasmatocoris papei View in CoL sp. nov. has an overall similarity not only to Ph. moraballi View in CoL but also to Ph. magdalenae Wygodzinsky, 1966 View in CoL and Ph. sturmi Wygodzinsky, 1966 View in CoL . These three latter species were considered as closely related by Wygodzinsky (1966), who also referred to them as “a group” of “dark, medium-sized species around [Ph.] magdalenae View in CoL ”, occurring in the Amazonian region. Phasmatocoris papei View in CoL sp. nov. also belongs to this group, but it can be separated from the other three species by the characteristics mentioned in the following key and specifically from Ph. moraballi View in CoL , which seems to be the most similar species, also by the differential diagnosis provided above.
Additionally, the phallic structures such as the shape of dorsal phallothecal plate and specially the processes of the endosoma are very different among Ph. magdalenae View in CoL , Ph. sturmi View in CoL and Ph. papei View in CoL sp. nov. ( Wygodzinsky 1966; Figs. 28– 29 View FIGURES 27–31 , 32–34 View FIGURES 32–34 ). Yet, the processes of endosoma of Ph. magdalenae View in CoL , Ph. moraballi View in CoL and Ph. sturmi View in CoL were recorded as strongly or clearly asymmetrical by Wygodzinsky (1966), while, as commented above, only slightly asymmetry was recorded in some of the structures of the endosoma of Ph. papei View in CoL sp. nov.
Interestingly, the entirely sclerotized ventral wall (as recorded in the paratype, Figs. 26 View FIGURES 25–26 , 30 View FIGURES 27–31 ) and the shape of the dorsal phallothecal plate ( Figs. 28–29 View FIGURES 27–31 ) of Ph. papei sp. nov. seem to be similar to those of Ph. praecellens ( Wygodzinsky 1966: figs. 86, L, O).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Emesinae |
|
Genus |
Phasmatocoris papei
| Gil-Santana, Hélcio R. 2018 |
Phasmatocoris papei
| Gil-Santana 2018 |
Phasmatocoris papei
| Gil-Santana 2018 |
Ph. papei
| Gil-Santana 2018 |
Ph. papei
| Gil-Santana 2018 |
Ph. moraballi
| Wygodzinsky 1966 |
Ph. magdalenae
| Wygodzinsky 1966 |
Ph. sturmi
| Wygodzinsky 1966 |
magdalenae
| Wygodzinsky 1966 |
Ph. moraballi
| Wygodzinsky 1966 |
Ph. magdalenae
| Wygodzinsky 1966 |
Ph. sturmi
| Wygodzinsky 1966 |
Ph. magdalenae
| Wygodzinsky 1966 |
Ph. moraballi
| Wygodzinsky 1966 |
Ph. sturmi
| Wygodzinsky 1966 |