CHALCOSIINAE, Walker, 1864
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2005.00139.x |
|
persistent identifier |
https://treatment.plazi.org/id/BA07932D-7117-FFD5-1896-F8EBCEECFF36 |
|
treatment provided by |
Diego |
|
scientific name |
CHALCOSIINAE |
| status |
|
THE CHALCOSIINAE View in CoL View at ENA
Amongst the zygaenid subfamilies, the Chalcosiinae s.l. are a diverse group. Second only in size to the Procridinae , they probably exhibit the highest diversity in morphology and ecology both within the Zygaenoidea and within the non-obtectomeran apoditrysian Lepidoptera . Owing to their often brilliant coloration, high level of sexual dimorphism, complicated mimetic patterns, little-known biology and rarity in museum collections, they have received the attention of many researchers and insect collectors. The group’s taxonomy has remained confusing since its initial documentation in the 18th century. Before Walker established the ‘Chalcosiidae’ in 1864, he ( Walker, 1854) placed several chalcosiine genera in unrelated families, e.g. Trypanophora Kollar, 1844 in Sesiidae & Pintia Walker, 1854 (= Cyclosia Hübner, 1860 ) in Lithosiidae (now Lithosiinae of Arctiidae ). In his original concept, only 19 genera were included ( Table 1); the subfamily subsequently increased in size and been associated with various unrelated lepidopteran families (e.g. Epicopeiidae , Geometridae , Arctiidae and Cossidae ; see Table 1).
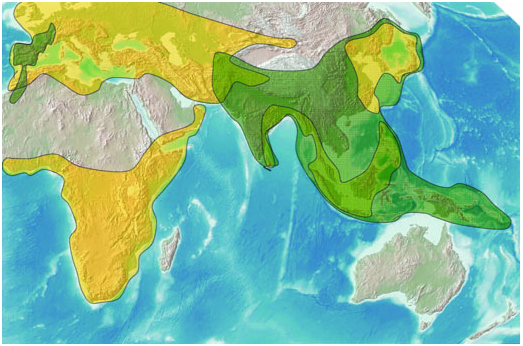
The subfamily currently comprises c. 70 genera and 370–400 species ( Bryk, 1936; Endo & Kishida, 1999; Fletcher & Nye, 1982; Tremewan, 1973; Yen, 2002c) and is distributed in an area ranging from Palaearctic eastern Asia, through subtropical south-east Asia, to the Melanesian and Micronesian archipelagos. It is unknown in Australia, New Zealand and the South Pacific islands. An isolated genus, Aglaope Latreille, Cotes & Swinhoe Hampson Jordan
Walker (1864) (1887) (1892) Kirby (1892) (1907–1909, 1907–1908) Hering (1922)
– Cadphises Cadphises Cadphises Cadphises Cadphises – – – Hampsonia Hampsonia
Aglaope 1 – Aglaope 1 – Aglaope – Philopator Philopator Philopator Philopator Philopator – Elcysma Elcysma Elcysma Elcysma – Agalope Agalope Agalope Agalope 17 Agalope 17 – Chelura Chelura 8 Achelura 8 – – – Boradia Boradia 9 Boradia Boradia Boradia – Campylotes Campylotes Campylotes Campylotes Campylotes – Herpa Herpa Herpa Herpa Herpa – – – – Panherpina – – – – Barbaroscia
Corma Corma Corma Corma Corma – Codane – Codane – – – Eucormiopsis – – – – – Docleomorpha – – – – Cryptophysophilus – – – Anarbudas Anarbudas
Cyclosia Cyclosia Cyclosia Cyclosia Cyclosia Cyclosia – Pintia Pintia 10 Pintia – –
Isbarta 2 Isbarta 10 Isbarta – – – Callamesia Callamesia 10 – – – – Epyrgis – Epyrgis 10 – – – – Klaboana 10 – – – – Mimeuploea 10 – –
Didina – Didina 10 – – – – – Rhodopsona Rhodopsona
Pidorus Pidorus Pidorus Pidoris Pidorus Pidorus – Laurion – Laurion* – – Arbudas Arbudas Arbudas * Arbudas Arbudas – Eumorphiopais
Fletcher (1925) Bryk (1936) Alberti (1954) Endo & Kishida
Tremewan (1973) (1999) Yen (2003a) Cadphises Cadphises Cadphises Hampsonia Hampsonia Hampsonia
Philopator Philopator Philopator
– –
Agalope Agalope 17 Agalope 17
– – Boradia Boradia Boradia Campylotes Campylotes Campylotes
Barbaroscia Barbaroscia Corma Corma Corma
Eucormiopsis Eucormiopsis
Cryptophysophilus Cryptophysophilus
Heterusinula Heterusinula Cyclosia Cyclosia Cyclosia Rhodopsona Rhodopsona Rhodopsona Pidorus Pidorus Pidorus
– –
– –
Kubia – Arbudas Arbudas Arbudas
– [not listed]
Thaumastophleps Thaumastophleps
Opisoplatia Opisoplatia Eterusia Eterusia Eterusia
Prosopandrophila Prosopandrophila Trypanophora Trypanophora Trypanophora Phlebohecta Phlebohecta Phlebohecta Pseudoscaptesyle Pseudoscaptesyle Pseudo [s]captesyle
Erasmiphlebohecta Erasmiphlebohecta Chalcophaedra Chalcophaedra Chalcophaedra Erasmia Erasmia Erasmia
Milleria Milleria Pseudonyctemera Pseudonyctemera Pseudonyctemera
Eusphalera Eusphalera Psaphis Psaphis Psaphis
Cadphises Cadphises Cadphises Hampsonia Hampsonia Hampsonia Herpidia Herpidia Herpidia
Philopator Philopator Philopator Formozygaena Formozygaena Formozygaena Atelesia [neglected] Atelesia
Boradia Boradia Boradia Campylotes Campylotes Campylotes Neoherpa 19 Herpa Neoherpa Panherpina Panherpina Panherpina Barbaroscia Barbaroscia Barbaroscia Corma Corma Corma Eucormiopsis Eucormiopsis Eucormiopsis Anarbudas Anarbudas Anarbudas Docleomorpha Docleomorpha Docleomorpha Cryptophysophilus Cryptophysophilus Cryptophysophilus Heterusinula Heterusinu [r]a Heterusinula Cyclosia Cyclosia Cyclosia Rhodopsona Rhodopsona Rhodopsona Pidorus Pidorus Pidorus
– Pseudarbudas Pseudarbudas Kubia 20 – – Arbudas Arbudas Arbudas Eumorphiopais Eumorphiopais Eumorphiopais Hemiscia Hemiscia Hemiscia
Cyanidia [neglected] Cyanidia Isocrambia [neglected] Isocrambia Herpolasia [neglected] Herpolasia Clematoessa [neglected] Clematoessa Hemichrysoptera [neglected] Hemichrysoptera Hadrionella [neglected] Hadrionella Sciodoclea Sciodoclea Sciodoclea Caprima [neglected] Caprima Allocaprima Allocaprima Allocaprima Thaumastophleps [neglected] Thaumastophleps Opisoplatia Opisoplatia Opisoplatia Eterusia Eterusia Eterusia
Soritia Soritia Soritia Mimascaptesyle Mimascaptesyle 22 – Prosopandrophila – 23 Prosopandrophila Trypanophora Trypanophora Trypanophora Phlebohecta Phlebohecta Phlebohecta Pseudoscaptesyle Pseudoscaptesyle Pseudoscaptesyle Erasmiphlebohecta Erasmiphlebohecta Erasmiphlebohecta Chalcophaedra Chalcophaedra Chalcophaedra Erasmia Erasmia Erasmia
Milleria Milleria Milleria Pseudonyctemera Pseudonyctemera Pseudonyctemera Eusphalera Eusphalera Eusphalera Psaphis Psaphis Psaphis
1809, with two sibling species confined to the west Mediterranean area, demonstrates an intriguing biogeographical disjunction from the other relatives ( Fig. 4 View Figure 4 ).
In contrast, unlike the western Palaearctic Zygaeninae (Burnet moths) and Procridinae (Forester moths, Smoky moths), which have been the subject of frequent and close study (e.g. Ebert, 1994; Guenin, 1997; Efetov & Tarmann, 1999; Naumann et al., 1999, De Freina & Witt, 2001), most of the previous studies dealing with the Chalcosiinae have restricted themselves to establishing new taxa and carrying out faunistic surveys.
The first described species, Chalcosia pectinicornis , was documented by Linnaeus from ‘Asia’ (possibly ‘southern China’) in 1758 (as Sphinx pectinicornis Linnaeus = Sphinx auxo Linnaeus, 1767 and Papilio (Heliconius) thallo Linnaeus, 1767 ) (see also Honey & Scoble, 2001: 385). Over the subsequent 250 years, the species diversity of this group was explored in the following works: Drury (1773); Cramer (1775 –1776), Fabricius (1775), Hübner (1816), Guérin-Méneville (in Delessert, 1843), Kollar (1844), Herrich-Shaffer (1850–1858), Walker (1854, 1856, 1864), Doubleday (1847), Butler (1877a, b), Moore (1878, 1879a, b, 1880– 1887), Snellen (1879), Druce (1888, 1896), Leech (1890, 1898), Hampson (1891, 1892), Swinhoe (1891, 1892, 1904), Kirby (1892), Oberthür (1893, 1894, 1896, 1910, 1923), Aurivillius (1894), Semper (1896 –1902), Dohrn (1899, 1906), de Joannis (1902, 1903), Piepers & Snellen (1903), Jordan (1907, 1908, 1912, 1923), Strand (1915, 1916), Eecke (1920, 1929), Hering (1922), Joicey & Talbot, 1922), Mell (1922), Matsumura (1927, 1931), Talbot (1926, 1929a, b), Bryk (1936, 1948), Inoue (1958, 1976a, b, 1982, 1987a, b, 1991, 1992), Lemée & Tams (1950), Kishida (1988, 1989a, b, 1995, 1996), Owada (1989, 1992a, b, 1996, 2001, 2002), Tarmann, 1992b, c), Horie, 1993, 1994a, b, Horie (1995), Yen (1996), Yen & Horie (1997), Yen & Yang (1997, 1998), Owada & Horie (1999, 2002a, b), Owada, Horie & Xue (1999), Endo & Kishida (1999), Horie & Awada (2000) and Horie et al. (2000). Nearly all the currently valid genera had been established by the 1940s, with only four genera added subsequently ( Yen & Yang, 1997, 1998; Efetov, 1999; Owada & Horie, 2002a).
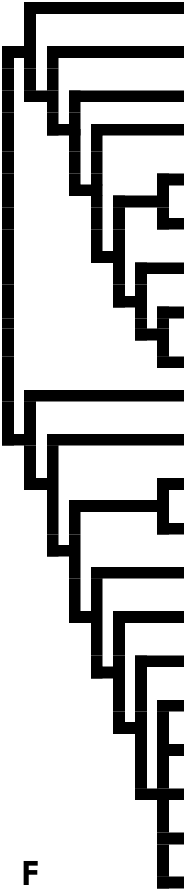
Hering (1922) was the first to survey the subfamily from a wider geographical perspective. Based largely on wing venation, shape and pattern, he proposed several new genera and provided comments on the relationships between them. His concept of Chalcosiinae included several genera that had already been transferred to the Procridinae (e.g. Procris Fabricius, 1807 , now a junior synonym of Adscita Retzius, 1783 ) and Callizygaeninae ( Procotes Butler, 1876 ). His ambiguous interpretation of characters resulted in some genera being poorly defined (e.g. Chalcosia Hübner, 1819 and Pidorus Walker, 1854 ; see Fig. 2). Subsequently, Bryk’s (1936) catalogue of Zygaenidae , which enumerated nearly all the synonyms and valid names in the Chalcosiinae known at that time, demonstrated the taxonomic difficulties and problems caused by phenetic and empirical taxonomic treatments since the Linnaean period.
In his world–wide review, Alberti (1954) used many character sets such as wing venation, genitalia and other external characters for grouping the subfamily. In his classification ( Fig. 1A View Figure 1 ), the Zygaenidae were divided into three major groups. Five tribes of the Chalcosiinae (Agalopini, Aglaopini , Cyclosiini, Chalcosiini and Heteropanini) were weakly defined and established for 40 examined genera, with 25 genera remaining unplaced owing to the unavailability of material ( Fig. 3 View Figure 3 ). All the generic names of the Zygaenidae published up to 1973, including those of the Chalcosiinae , were revised and verified by Tremewan (1973), whose catalogue was followed by that of Fletcher & Nye (1982). For major changes in the composition of Chalcosiinae during the last 150 years see Table 1.
Since the 1980s, concomitant with the rapid increase in trade in insects within south-east Asia, new taxa have been described and faunal surveys conducted of this diurnally active subfamily. However, since the supraspecific levels of the Chalcosiinae have not been revised using modern techniques, and since none of the postulated synapomorphies has been verified based on an overall survey, species-level studies face considerable problems when attempting to allocate new species to the correct genera. In addition, the mimetic wing patterns and high degree of sexual dimorphism have led to conflict between character sets, making for problematic classification and tentative taxonomic treatment.
Tarmann (1992a) suggested that the hindwing/ abdominal androconial system found in the Arbudas - complex would make a good starting point for accessing the potential apomorphies of the Chalcosiinae . Following this proposal, Epstein et al. (1999; see also Naumann, 1988; Naumann et al., 1999) recognized three potential apomorphies for Chalcosiinae : (1) male with an androconial organ at base of hindwing and abdominal pleurite; (2) female without a pair of secondary accessory glands close to ooporus; (3) terminal segments in female form a functional ovipositor. However, since these three characters have been found to be inapplicable to many genera, using them to support the monophyly of the Chalcosiinae seems to be questionable ( Yen & Yang, 1997; Yen, 2003c). While investigating the family-level phylogeny of Zygaenoidea, Naumann & Feist (1987) and Fänger & Naumann (1998, 2001) uncovered several ultrastructural characters that may have considerable phylogenetic significance, but which need to be surveyed across the whole of Zygaenoidea or Zygaenidae .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |