Sinoscarterella incompleta, Wang & Nel & Fu & Su & Cai & Liu & Gao & Huang, 2022
|
publication ID |
https://doi.org/ 10.11646/palaeoentomology.5.2.12 |
|
publication LSID |
lsid:zoobank.org:pub:D5677074-3D13-4D8A-8B9C-03BE43E1DA47 |
|
DOI |
https://doi.org/10.5281/zenodo.6532898 |
|
persistent identifier |
https://treatment.plazi.org/id/03CBDC45-FFF1-7B0C-33E7-FE45FB19DE7D |
|
treatment provided by |
Plazi |
|
scientific name |
Sinoscarterella incompleta |
| status |
sp. nov. |
Sinoscarterella incompleta sp. nov.
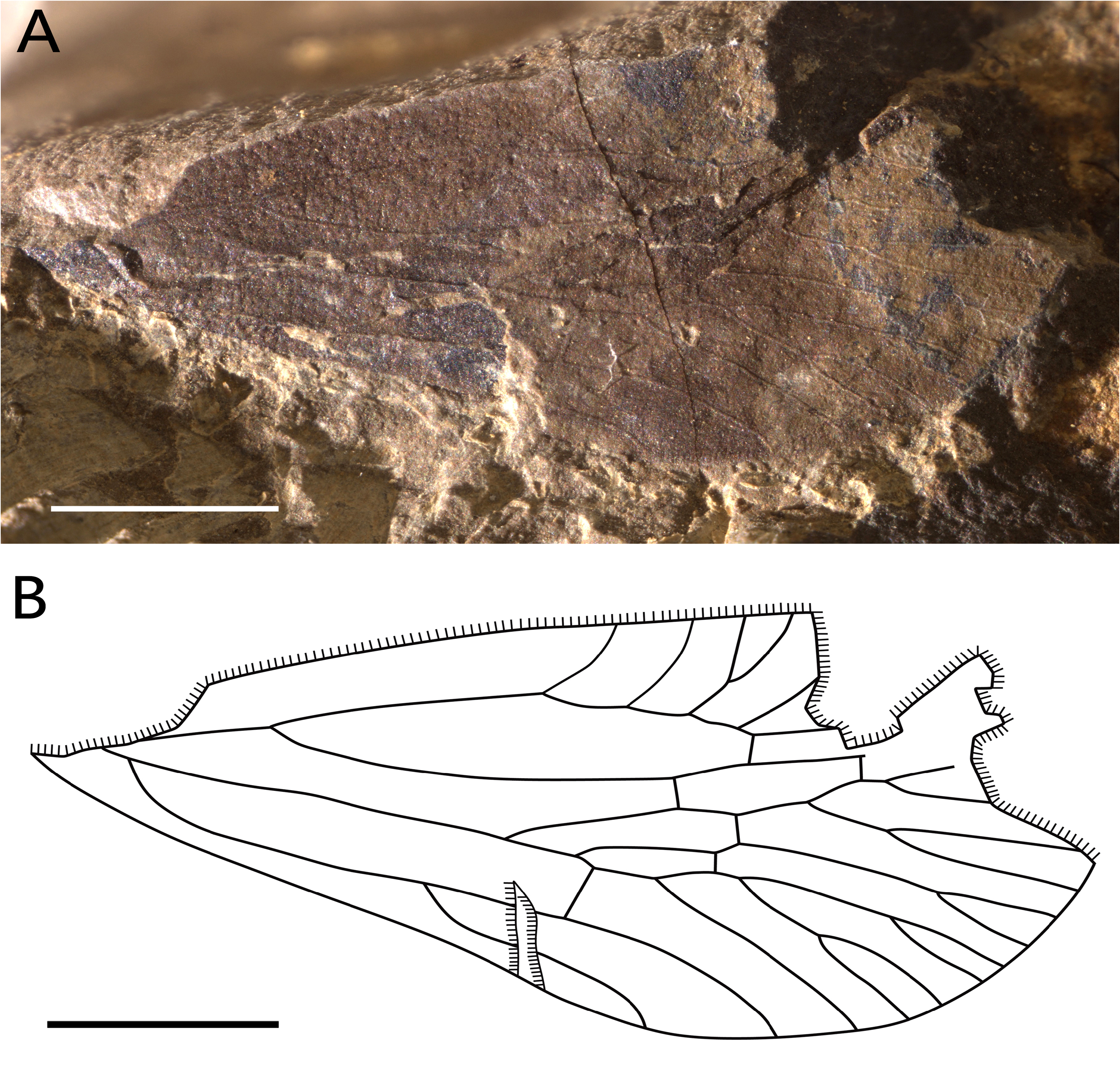
( Fig. 4 View FIGURE 4 ) urn:lsid:zoobank.org:act:CD4091CC-A2E9-4C0F-8EA3-E4DCE6D7DD4D
Material. Holotype, NIGP180151 View Materials , an incomplete isolated forewing.
Etymology. Named after the incomplete state of preservation of the fossil.
Diagnosis. As for the genus.
Type locality and horizon. A fossil locality near the Daheyan Town, Turpan City, Xinjiang; late Early Jurassic .
Description. Forewing rugose and punctate in areas between C and R and RA and RP, evenly rounded, with basal portion narrowed; preserved part 8.3 mm long, wing 4.2 mm wide, no pc vein between Costa and R; postclaval portion of membrane (tornus) curved, postnodal membrane distinctly widened; RA smoothly curved with five anterior branches, third one being forked again; no anterior branch of R basad base of RP; RA1 entering anterior margin before level of claval apex; RP simple, separated from common stalk ScP+R at basal ⅓ of tegmen length; base of RP well distad base of MP+CuA; stems MP and CuA not forking at same level; MP with 11 terminals; veinlet ir (ra-rp) present, sometimes shortened; two veinlets rp-mp and one mp-cua present; branches of CuA elongate.
Remarks. This fossil is a forewing of a cicadomorphan ( Hemiptera ) because of the tegminisation of the anterior part of wing and the general pattern of venation. The ‘base of RP far distad bases of MP and CuA’ and the ‘basal to sub-basal fusion of ScP with R’ are characters present in taxa of few Permian and/or Triassic families, viz. some Prosbolidae , Cicadoprosbolidae Evans, 1956 , Saaloscytinidae Brauckmann , Martins- Neto & Gallego, 2006, Maguviopseidae Shcherbakov, 2011 , Mesojabloniidae Storozhenko, 1992 , Pereboriidae Zalessky, 1930 , Magnacicadidae Hong & Chen, 1981, Dysmorphoptilidae Handlirsch, 1906 , Hylicellidae Evans, 1956 , and Scytinopteridae Handlirsch, 1906 . Indeed, a global phylogenetic analysis of all these groups would be necessary to make precise their definitions and limits (see catalogue in Szwedo, 2018).
The Saaloscytinidae and Maguviopseidae have forewing tegminised on all their surface and no distal anterior veinlets between RA and C ( Shcherbakov, 2011). Some Prosbolidae ( Orthoscytina spp. ) and Scytinopteridae ( Triassoscytinopsis Evans, 1956 ) could show similarities with the new fossil, in the presence of a distal series of veinlets between RA and Costa and a simple RP, but the area between RA and RP is much narrower in these taxa than in the new fossil, and the basal-most such veinlet (ScP re-emerging from RA?) is different from the more distal ones, stronger and/or aligned with basal part of RA, unlike in the new fossil.The Magnacicadidae have rounded forewings with a broader area between R/RA and C and a different pattern of branches of the main veins ( Hong & Chen, 1981). Pereboriids have much more branches of MP and RP than the new fossil. Some cicadoprosbolids ( Cicadoprosbole Evans, 1956 ) and some mesojabloniids also share with the new fossil the presence of a distal series of veinlets between RA and Costa but they have a distinct elongate vein pc ( Evans, 1956; Shcherbakov, 2011).
Shcherbakov (1988a, b), followed by Nicholson et al. (2015) and Szwedo & Huang (2019), synonymized the Eoscarterellidae under Dysmorphoptilidae , but the former was treated as a family by the other authors ( Evans, 1956; Carpenter, 1992; Hamilton, 1992; Szwedo et al., 2004; Jell, 2004; Szwedo, 2018). Among Dysmorphoptilidae , Eoscarterellinae Evans, 1956 have forewing venations most similar to that of the new fossil, viz. apical portion of tegmen not abruptly narrowed; postclaval margin (tornus) arcuate, convex, not sigmoidal (characteristic of the Eoscarterellinae after the key to dysmorphoptilid subfamilies in Szwedo & Huang, 2019: 153); bSc shortened and ending into R basad the re-emergence of MP+CuA; fork of CuA very deep and narrow with elongate branches; MP with several distal branches; base of RP far distad base of MP+CuA; RP simple, an elongate and broad cell between RA and RP; area between RA and RP distinctly narrowed in its distal half; and RA with a distal series of curved veinlets between RA and Costa.
Szwedo and Huang (2019: 159) proposed the following diagnosis for Eoscarterellinae : tegmen rugose, punctate, evenly rounded, basal portion narrowed; postclaval portion of membrane (tornus) arcuate, postnodal membrane distinctly widened; branch ScP+RA with more than two branches; RA 1 entering anterior margin before level of claval apex; RP separated from common stalk ScP+R at basal ⅓ of tegmen length; veinlet ir (ra-rp) present, sometimes shortened; veinlets rp-mp and mpcua present. All these characters are present in the new fossil. Only the character ‘MP with four terminals’ is not present, as the new fossil has 11 terminals, and four main branches.
Eoscarterellinae currently comprise the genera Eoscarterella Evans, 1956 (Late Triassic, Australia), Belmontocarta Evans, 1958 (Late Permian, Australia), Duraznoscarta Lara & Wang, 2016 (Late Triassic, Argentina), Dysmorphoscartella Riek, 1973 (Late Permian, South Africa), and Eoscartoides Evans, 1956 (= Mesonirvana Evans, 1956 ; Evans, 1956; Late Triassic, Australia). Eoscarterella has branches of CuA shorter and much less branches of MP than in the new fossil; Eoscartoides differs from the new fossil in the basal cell closed with short basal portion of stem CuA (‘arculus’) and stems R and MP leaving basal cell at same point, and less numerous branches of MP and the forked most basal anterior branch of RA ( Evans, 1956; Lambkin, 2016). Duraznoscarta has branches of R basad the emergence of RP and much more anterior branches of RA ( Lara & Wang, 2016). Dysmorphoscartella lobata Riek, 1973 strongly differs from the new fossil and the other Eoscarterellinae in the presence of an elongate and pectinate first branch of RA+ScP and the postclaval portion of membrane nearly straight ( Riek, 1973: fig. 13). Belmontocarta perfecta Evans, 1958 resembles the new fossil, with the following differences: the stems R and MP leaving basal cell at same point vs. stems MP and CuA leaving basal cell at same point, the third veinlet between RA and C is simple vs. forked in the latter, first anterior branch of MP forked basad crossvein rp-mp vs. distad in the latter, first posterior branch of MP forked distad crossvein mp-cua vs. basad in the latter, first posterior branch of MP with only two branches vs. seven along posterior wing margin in the latter ( Evans, 1956). Both fossils share the presence of punctuation in the area between C, R, RA and RP.
The forewing venations of the species of the hylicellid genus Cycloscytina Martynov, 1926 (Vietocylinae Shcherbakov, 1988) strongly resemble that of the new fossil in the veinlets between RA and C, a crossvein imp present, making the cell C3 closed, and two crossveins rp-mp, making the cell C2 present (as in Vietocycla Shcherbakov, 1988 , but unlike the other Hylicellidae Evans, 1956 that have only one or two short crossveins between RA and C (see Evans, 1956; Shcherbakov, 2011, 2012), the shape of the basal cell between RA and RP, two crossveins between RP and MP. Vietocycla strongly differs from Cycloscytina and the new fossil in the presence of anterior branches of R basad emergence of RP and between base of RP and posterior curvature of RA, and in first branch of RA at level of the beginning of this curvature with three branches. The pattern of R/RA in Vietocycla is similar to that of Duraznoscarta .
Cycloscytina delutinervia Martynov, 1926 (Late Jurassic) differs from the new fossil by the less numerous branches of MP and shorter branches of CuA, but Cycloscytina fulgoroides ( Becker-Migdisova, 1962) (Triassic,originallyin Asiocixius Becker-Migdisova,1962 , a genus synonymized with Cycloscytina by Shcherbakov, 1988, but see also Szwedo et al., 2004), has much more branches of MP (even more than in the new fossil) and longer branches of CuA, but supposedly no crossvein ir and only one crossvein rp-mp. Cycloscytina fulgoroides also differs from the new fossil in the aligned forks of MP and CuA. Cycloscytina asiatica ( Martynov, 1937) (= Mesocixiella rohdendorfi Becker-Migdisova, 1962 ) (Early Jurassic), and Cycloscytina extensa ( Martynov, 1937) (Early to Middle Jurassic ), Cycloscytina korlaensis ( Hong, 1983) (Middle Jurassic) , and Cycloscytina gobiensis (Shcherbakov, 1988) ( Middle to Late Jurassic), have also numerous anterior branches of RA, and share with the new fossil a posterior curvature of main stem of RA. All differ from the latter in the less numerous branches of MP, and the fork of CuA distad the level of that of MP ( Martynov, 1937; Becker-Migdisova, 1962; Hong, 1983; Shcherbakov, 1988a, b).
Given the traits discussed above, we tentatively placed the fossil in Eoscarterellinae , and raised some questions about taxonomic units. The venation of the Cycloscytina spp. resembles those of the Eoscarterellinae , to the point that we could not find any significant differences, except for the number of branches of RP and MP, characters variable in the genus Cycloscytina and the Eoscarterellinae . It is likely possible that Cycloscytina belongs to the Eoscarterellinae , but only a phylogenetic analysis could help to solve the problem.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
InfraClass |
Lower |
|
SuperOrder |
Odonatoptera |
|
Order |
|
|
Family |
|
|
SubFamily |
Eoscarterellinae |
|
Genus |