Caridina thomasi, Rintelen & Karge & Klotz, 2008
|
publication ID |
https://doi.org/ 10.1080/00222930802254680 |
|
persistent identifier |
https://treatment.plazi.org/id/F66D932E-3918-C73B-FD95-B735FD84F93B |
|
treatment provided by |
Felipe |
|
scientific name |
Caridina thomasi |
| status |
sp. nov. |
Caridina thomasi View in CoL sp. nov.
( Figures 2 View Figure 2 , 3 View Figure 3 )
Material examined
Holotype. Female, CL 4.7 mm ( MZB Cru 1682), Indonesia, Central Sulawesi, Banggai Islands, Peleng , west peninsula, east of Alani , river with lake-like extension, (01 ° 28.3159S 122 ° 52.4739E; collected by K. and T. von Rintelen, 25 September 2005). GoogleMaps
Paratypes. Sixteen females, CL 3.0– 5.3 mm ( MZB Cru 1683), same data as holotype GoogleMaps ; 14 males, CL 3.4–4.5 mm ( MZB Cru 1683), same data as holotype GoogleMaps . 15 females, CL 2.8–4.9 mm ( ZMB 29413), same data as holotype GoogleMaps ; three ovigerous females, CL 4.6– 5.1 mm ( ZMB 29413), same data as holotype GoogleMaps ; 13 males, CL 3.4–4.3 mm ( ZMB 29413), same data as holotype GoogleMaps .
first pereiopod; (L) second pereiopod; (M) uropodal diaeresis; (N). scanning electron micrograph of chela and carpus of first and second pereiopods; (O) preanal carina, paratype male (CL 3.8 mm; MZB Cru 1683); (P) endopod of male first pleopod (ZMB 29413); (Q) appendix masculina of male second pleopod. Scale bars: A, B, P, Q51.0 mm; C–E, K– O 50.5 mm; H–J 52.2 mm; F, G 50.1 mm.
Comparative material examined
Caridina typus H. Milne Edwards, 1837. One ovigerous female, CL 8.7 mm ( ZMB 29092), with over 100 eggs, egg size 0.40–0.45× 0.24–0.29 mm (n 520, eggs without eyes), Indonesia, South Sulawesi, Maros karst, Bantimurung , above the waterfall, 05 ° 0.969S 119 ° 40.929E GoogleMaps ; collected by K. and T. von Rintelen, 12 October 2003. One ovigerous female, CL 8.2 mm ( ZMB 29019), with over 100 eggs, egg size 0.38– 0.43× 0.24–0.26 mm (n 510, eggs without eyes), Indonesia, Southeast Sulawesi, stream north of Abola, road Tinobu-Kendari , 03 ° 40.9959S 122 ° 15.2989E; collected by M. Glaubrecht GoogleMaps , 1 June 2005. One ovigerous female, CL 5.9 mm ( ZMB 29206), with over 100 eggs, egg size 0.41–0.44× 0.25–0.26 mm (n 510, eggs with eyes), Indonesia, Southeast Sulawesi, river at road Malili-Tolala , 02 ° 52.59S 121 ° 4.759E GoogleMaps ; collected by K. and T. von Rintelen, 12 September 2003. Six females, CL 3.8–7.0 mm ( ZMB 29344), Indonesia, Bali, South Bali, stream north of Antap, tributary of Yehotan River , 08 ° 30.7469S 115 ° 1.379E; collected by M. Glaubrecht GoogleMaps and K. and T. von Rintelen; one male CL 4.2 mm ( ZMB 29344), same data as females. One ovigerous female, CL 7.4 mm ( ZMB 29409), with over 100 eggs, egg size 0.40– 0.42× 0.25–0.28 mm (n 510, eggs without eyes), Indonesia, Moluccas, Halmahera, river at road Sidangoli-Tobelo , 00 ° 58.8799N 127 ° 37.429E; collected by K. von Rintelen GoogleMaps , 19 September 2005; one female CL 7.7 mm ( ZMB 29409), same data as ovigerous female GoogleMaps .
Description
Cephalothorax and cephalic appendages. Carapace length 2.8–5.3 mm (median 4.0 mm). Rostrum straight and short ( Figure 2A,B View Figure 2 ), not reaching to basal segment of antennular peduncle and not reaching beyond length of eyes, 0.2–0.3 (median 0.2) times as long as carapace, completely unarmed, only one male with one dorsal tooth at half length of rostrum (MZB Cru 1683). Inferior orbital angle fused with indistinct antennal spine. Pterygostomian angle broadly rounded. Eyes well developed with cornea globular, anterior end reaching two-thirds of basal segment of antennular peduncle. Antennular peduncle 0.5–0.7 (median 0.6) times as long as carapace (n 558), second segment 1.1–2.0 times length of third segment (n 531), third segment 0.2–0.5 times length of basal segment (n 529). Stylocerite reaching well beyond halflength of basal segment of antennular peduncle, but not reaching end of it. Scaphocerite ( Figure 2E View Figure 2 ) 2.7–2.9 times as long as wide (n 55).
Abdominal somites, telson and uropods. Sixth abdominal somite 0.4–0.6 (median 0.5) times length of carapace, 1.6–2.1 (median 1.8) times as long as fifth somite, about as long as telson. Telson ( Figure 2H–J View Figure 2 ) 2.5–3.1 (median 2.6) times as long as proximally wide (n 521), distal margin broadly rounded and slightly flattened, without median projection, with three to five pairs of dorsal spinules and one pair of dorsolateral spinules; distal end with four to eight spines, lateral pair distinctly longer than intermediate spines. Preanal carina rounded, lacking a tooth or spine with few setae ( Figure 2O View Figure 2 ). Uropodal diaeresis ( Figure 2M View Figure 2 ) with 13–15 movable spinules (n 511).
Mouthparts and branchiae. Incisor process of mandible ending in irregular teeth, molar process truncated ( Figure 3D View Figure 3 ). Lower lacinia of maxillula broadly rounded, upper lacinia elongate, with numerous distinct teeth on inner margin, palp slender with few simple setae at tip ( Figure 3E View Figure 3 ). Upper endites of maxilla subdivided, palp short, scaphognathite tapering posteriorly, fringed with long, curved setae at posterior margin ( Figure 3F View Figure 3 ). Palp of first maxilliped ending in a broadly triangular projection ( Figure 3G View Figure 3 ). Podobranch on second maxilliped reduced to a lamina with few finger-like projections ( Figure 3H View Figure 3 ). Third maxilliped with one arthrobranch, ultimate segment as long as or slightly shorter than penultimate segment ( Figure 3I View Figure 3 ). Pleurobranchs present on all pereiopods. First pereiopod without arthrobranch. Well-developed epipods present on third maxilliped and first four pereiopods.
Pereiopods. Chela and carpus of first pereiopod distinctly stouter and broader than chela and carpus of second pereiopod ( Figure 2K, L and N View Figure 2 ); chela of first pereiopod 1.8–2.1 as long as wide (n 519), 1.3–2.1 times length of carpus; tips of fingers rounded, without hooks; dactylus 0.7–2.0 as long as palm (n 519); carpus 1.4–1.9 times as long as wide, 0.9–1.3 times length of merus (n 516). Chela of second pereiopod 2.2–2.8 times as long as wide (n 56), 0.8–0.9 times length of carpus; tips of fingers rounded, without hooks, dactylus 1.0–1.8 times as long as palm (n 523); carpus 3.8–5.4 times as long as wide (n 518), about as long as or slightly longer than merus. Third pereiopod slender ( Figures 2C, F View Figure 2 ; 3C View Figure 3 ), dactylus 2.7–3.4 times as long as wide (terminal spine included, without spines on flexor margin; n 56), terminating in one large claw with five to seven accessory spines on flexor margin; propodus 8.8– 11.7 times as long as wide, 3.9–4.7 times as long as dactylus; carpus 5.1–5.8 times as long as wide, 0.6–0.7 times as long as propodus, 0.5–0.6 times as long as merus; merus 5.4–6.5 times as long as wide, 1.7–2.0 times as long as carpus, bearing four to six (distal three to five densely spaced) strong, movable spines on posterior margin of outer surface. Distal three to five spines remarkably densely spaced. Fifth pereiopod slender ( Figure 2D,G View Figure 2 ), dactylus 3.1–4.3 times as long as wide (terminal spine included, without spines on flexor margin; n 56), terminating in one large claw with 33–48 spinules on flexor margin; propodus 10.6–13.3 times as long as wide, 3.3–4.6 times length of dactylus, carpus 5.4–6.0 times as long as wide, 0.5–0.6 times as long as propodus, 0.7 times as long as merus; merus 6.0–6.9 times as long as wide, 1.4–1.5 times length of carpus, bearing one or two strong, movable spines on posterior margin of outer surface.
Pleopods. Endopod of male first pleopod ( Figures 2P View Figure 2 ; 3A View Figure 3 ) elongated, triangular, 2.4– 3.4 times as long as proximal width (n 56), 0.4–0.5 times as long as exopod, without appendix interna. Appendix masculina on male second pleopod ( Figures 2Q View Figure 2 ; 3B View Figure 3 ) slender, rod-like, with long spines on inner and distal margin, appendix interna reaching to about two-thirds of appendix masculina.
Reproductive biology. Ovigerous females with 12– 20 eggs (n 53 females); egg size 1.10–1.27× 0.71–0.88 mm (n 545, eggs without eyes; sex ratio females: males 1.3; ratio females: ovigerous females 10.7).
Distribution. Caridina thomasi is only known from Peleng Island.
Habitat. Karstic freshwater river with a lake-like extension at an altitude of approximately 370 m. Mainly rocks in shallow water with little vegetation. Shrimps were found everywhere, but primarily in crevices of rocks along the shore.
Body colouration of living specimens. Body and appendages bright orange, partly red or yellow (K. and T. von Rintelen, personal field observation).
Etymology. Caridina thomasi is dedicated to Thomas von Rintelen, who not only accidentally discovered the new species with the first author while looking for new snail species, but who also greatly supports the Indonesian shrimp project.
Remarks
So far, C. thomasi is the only freshwater shrimp known from Peleng Island. The small number of eggs and their large size (. 1 mm, n 512 to n 520) as well as the typical freshwater habitat suggest a direct larval development indicative of a local endemism on Peleng Island, similar to other endemic species from Sulawesi (e.g. Zitzler and Cai 2006; Cai and Wowor 2007). In contrast, the numerous and comparatively small eggs (, 0.5 mm, n.100) of C. typus are characteristic of a prolonged larval development typical for widespread species (e.g. Benzie 1982; Hayashi and Hamano 1984).
With regards to the short, unarmed rostrum C. thomasi resembles the ubiquitous species C. typus, but both can easily be separated by their reproductive biology (see above), by the denticulation of the rostrum (ventrally unarmed in C. thomasi vs. 0– 5(2) ventral teeth in C. typus), the rounded tip of the dactylus of the first pereiopod (vs. with a prominent hook in C. typus), the reduced gill formula (see below) and the body size (CL 2.8–5.3 mm, median 4.0 mm, in C. thomasi vs. 5.9–8.7 mm, median 5.2 mm, in C. typus, the largest representative of the genus). Among other species of the genus Caridina with an abbreviated or direct larval development C. thomasi resembles C. cebuensis based on its original description from Cebu, Philippines ( Cai and Shokita 2006), but differs from this species in its broadly rounded pterygostomian angle of the carapace (vs. subrectangular in C. cebuensis ), in the shape of the male’s first pleopod (elongated triangular, 0.4–0.5 times as long as exopod vs. subrectangular, 0.25 times as long as exopod in C. cebuensis ) and the reduced branchiae.
Caridina thomasi can be further distinguished from C. typus, C. cebuensis and likewise from all other currently described species of the genus Caridina by its characteristic densely arranged spines on the merus of the third and fourth pereiopod (vs. not densely arranged in other species).
Conspicuous characteristics of C. thomasi are the reduced branchiae that rather resemble members of the genus Lancaris Cai & Bahir 2005 and have not been described in Caridina so far: the arthrobranch absent on the first pereiopod, the third maxilliped with only one arthrobranch and the podobranch on the second maxilliped reduced to a lamina. However, from the two currently described species of the genus Lancaris , C. thomasi differs in the densely arranged distal spines on the merus of the third and fourth pereiopod, the spines on the distal margin of the telson (intermedial plumose setae shorter than lateral pair vs. longer in Lancaris ), the uropodal diaeresis (lateral angle about as long as mesial movable spines vs. shorter in Lancaris ) and the absence of an appendix interna on the male first pleopod.
Molecular results
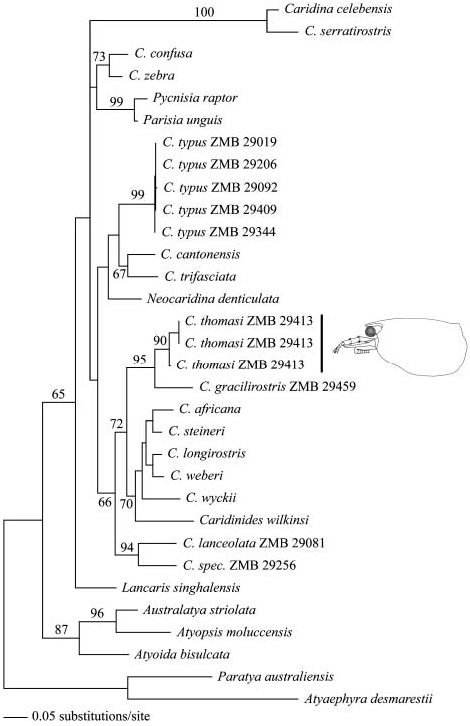
The ML analysis recovered three trees of equal likelihood (– ln L54125.87390), of which the first one is shown here ( Figure 4 View Figure 4 ). Differences in tree topology were only found in the branching order of the C. typus sequences, which are almost identical.
The molecular phylogeny places C. thomasi within a well-supported clade of typical species of Caridina : C. africana Kingsley 1882 , C. longirostris H. Milne Edwards 1837 , C. weberi de Man 1892 and C. wyckii (Hickson 1888) , including the cave species C. steineri Cai 2005 from Madagascar and the genus Caridinides Calman 1926 . Despite considerable morphological differences the sister taxon of C. thomasi is the ubiquitous species C. gracilirostris , a grouping highly supported by ML bootstrap values. The three specimens of C. typus from Sulawesi that were also morphologically examined and the two specimens from Halmahera and Bali are not closely related to C. thomasi , but appear in a separate clade with C. cantonensis Yü 1938 and C. trifasciata Yam & Cai 2003 . The two endemic species from Sulawesi, C. lanceolata and C. spec., are also not closely related to C. thomasi . The genus Lancaris is basal to all sequenced species of Caridina and some other atyid genera, such as Pycnisia Bruce 1992 , Parisia Holthuis 1956 or Neocaridina Kubo 1938 . This topology is not supported by ML bootstrap values, Lancaris and C. thomasi are clearly shown to be genetically distinct. For further details and discussion about the majority of already published sequences compare Page et al. (2007a, 2007b).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |