Cyrtogenius luteus (Blandford, 1894)
|
publication ID |
https://doi.org/ 10.5281/zenodo.215247 |
|
DOI |
https://doi.org/10.5281/zenodo.6180783 |
|
persistent identifier |
https://treatment.plazi.org/id/039C8797-B15D-6B66-FF34-FD81588DF9FE |
|
treatment provided by |
Plazi |
|
scientific name |
Cyrtogenius luteus (Blandford, 1894) |
| status |
|
Cyrtogenius luteus (Blandford, 1894) View in CoL
Dryocoetes luteus Blandford Carposinus pini Hopkins
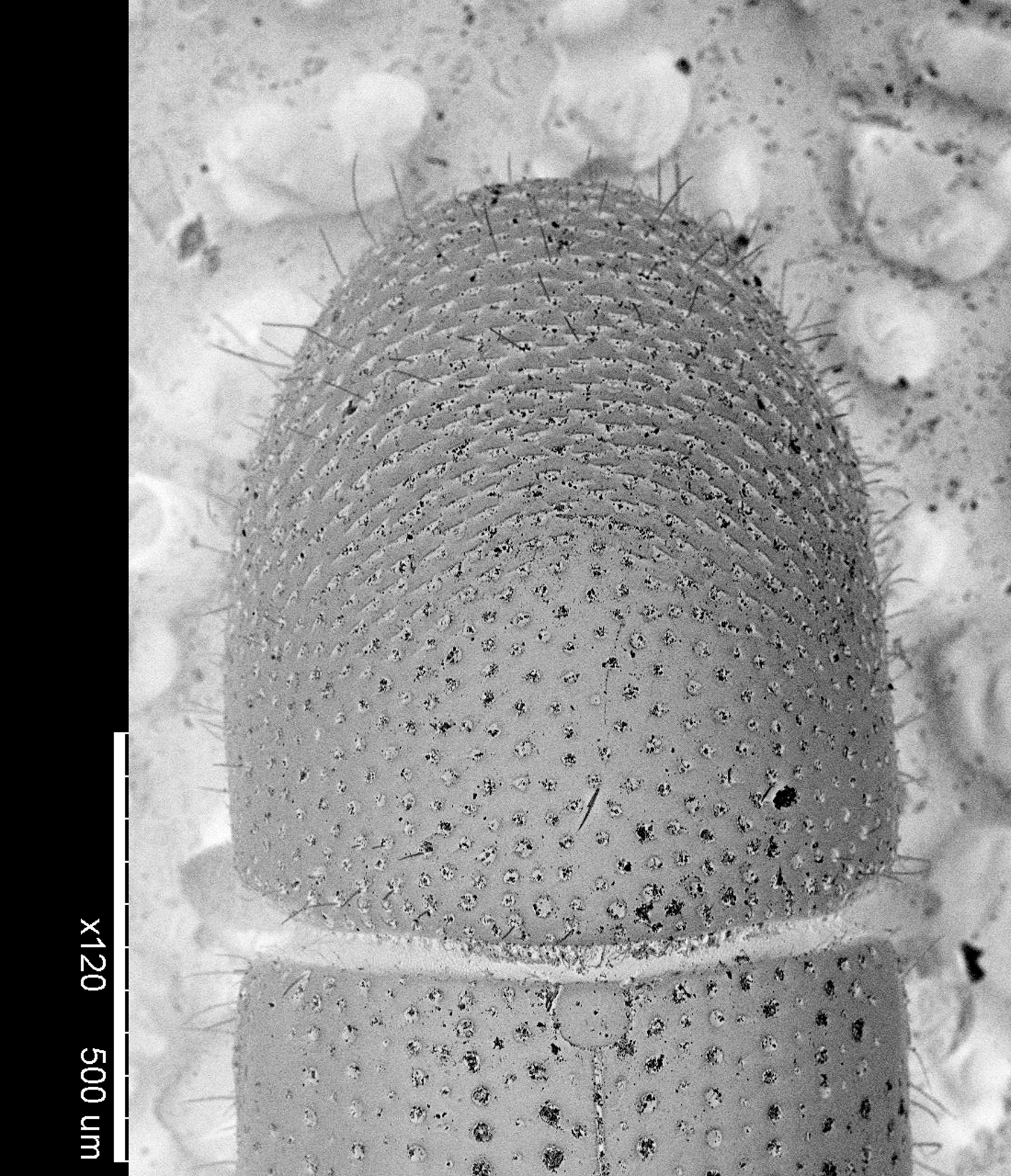
General features. Cyrtogenius luteus ( Fig. 1), the only Cyrtogenius species so far known to Europe, is similar to the European species belonging to the genus Dryocoetes from which is easily distinguished by the 4-segmented antennal funicle ( Fig. 2 View FIGURE 2 ), and the slightly elevated ventro-lateral margin of the declivity which is armed by small granules ( Figs. 1 and 3 View FIGURE 3 ). Other features concern the antennal club, which has a basal corneous portion (segment 1) always procurved, and sutures visible only on the anterior face ( Fig. 2 View FIGURE 2 ). Compared to the other Dryocoetini genera, Cyrtogenius luteus is a stout species with the elytrae 1.4 times longer than the pronotum ( Fig. 1).
Diagnosis. Female. Body cylindrical and shiny, brown ( Fig. 1). Body length 2.28 ± 0.045 mm (mean ± SEM, n = 10); 2.74 ± 0.041 longer than wide. Frons weakly convex, transversely impressed just above the epistoma; surface closely granulate-punctate with abundant short vestiture ( Fig. 5 View FIGURE 5 ). Eyes broadly emarginated. Antennal funicle 4-segmented ( Fig. 2 View FIGURE 2 ). Pronotum 1.09 ± 0.081 times longer than wide ( Fig. 4 View FIGURE 4 ); sides straight and sub-parallel on basal half; anterior margin broadly rounded; apical half finely and closely asperate; posterior half shiny with coarse and deep punctures, spaced by three times the diameter of a puncture; summit indistinct; hair-like setae mostly on asperate surface as well as on the posterolateral part ( Fig. 4 View FIGURE 4 ); lateral margins on basal two-thirds acutely margined. Elytra shiny, 1.57 ± 0.026 longer than wide, 1.4 ± 0.055 times longer than pronotum, sides straight and sub-parallel on basal three-fourths, posteriorly terminating broadly rounded, almost squared ( Fig. 1); discal striae indistinctly impressed; striae l and 2 impressed near declivity, marked by large and deep punctures; interstriae slightly wider than striae with close punctures of smaller size. Declivity very steep with convex face; striae l, 2 and 3 sulcate; stria l more strongly impressed than on disc, interstria l moderately elevated; interstriae l, 2 and 3 wider than on disc, each bearing an uniseriate row of widely spaced pointed granules; interstriae 4–7 bearing granules, those on 7 larger. Vestiture of fine hairs both on elytra and declivity; disc devoid of hairs.
Male. Similar to female in all morphological characters, except in having frons with finer punctures on smooth surface and with inconspicuous hairs ( Fig. 6).
Distribution. The species is so far known from different parts of the Oriental, Papuan and Oceanian regions, including India, Burma, China (Fujian, Guangdong, Guangxi, Henan, Hunan, Jiangsu, Jiangxi, Shanxi, Sichuan, Yunnan), Japan, Korea, Thailand, Myanmar, Taiwan, Malaysia, Indonesia (Java), Philippines, Micronesia, Bismarck Islands, Caroline Islands, Gilbert Islands, Mariana Islands, Samoa and New Guinea ( Wood & Bright 1992; Beaver & Liu 2010).
Host trees. Species usually breeding in the phloem of conifers, especially Pinus ( P. densata , P. k h a s y a, P. m a s - soniana, P. tabulaeformis , and P. yunnanensis ), but also recorded from other Pinaceae ( Larix and Picea ) ( Wood & Bright 1992; Beaver & Liu 2010). In Italy it has been found only in traps.
Genetic analysis. Cyrtogenius luteus and C. brevior are very similar species and there are no keys available for distinguishing them morphologically. C. luteus has been recorded from coniferous hosts and it has a more northerly distribution, whereas C. brevior is known from angiosperm hosts and has a southerly distribution (Beaver, pers. comm.). To separate these two sibling species, since we did not know the host trees, we performed a genetic analysis of the adults found in the traps. Some individuals freshly collected from traps were singly stored in 90% ethanol until DNA extraction. Nucleic acid extraction was performed on single specimens following a salting out protocol ( Patwary et al. 1994). A segment of the mitochondrial COI gene was amplified using the universal primers HCO1490 and LCO2198 ( Folmer et al. 1994). DNA sequencing was performed by dideoxy chain termination method at BMR Genomics sequencing service (BMR Genomics srl, Padova, Italy). Sequences were then deposited in GenBank (accession numbers JQ417240 View Materials , JQ417241 View Materials , JQ417242 View Materials , JQ621833 View Materials , JQ621834 View Materials ). Using MEGA version 5 ( Tamura et al. 2011) sequences were then aligned with all the COI sequences in GenBank obtained from specimens belonging to the genus Cyrtogenius : a sequence isolated from C. brevior − collected in Papua New Guinea ( Cognato et al. 2011), GenBank #s HM064152 View Materials − and two sequences of C. chirindaensis (GenBank #s AF187118 View Materials and AF438511 View Materials ). Moreover, a phylogenetic tree was reconstructed with the NJ method (Kimura-two parameters distance) using Dryocoetes autographus as outgroup (GenBank ID: AF444054 View Materials ). Node support was assessed by performing 1000 bootstrap replicates.
The comparison of the sequences obtained from our samples with the sequences of the other two Cyrtogenius species clearly shows that our samples belong to a species different from C. brevior although closely related to it ( Fig. 7 View FIGURE 7 ). In addition, the analysed samples show five different haplotypes between years and localities (Tab. 2); the consistent number of haplotypes may suggest a large single introduction.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.