Austroconops annettae Borkent, 2004
|
publication ID |
https://doi.org/ 10.1206/0003-0082(2004)449<0001:AWALAL>2.0.CO;2 |
|
DOI |
https://doi.org/10.5281/zenodo.5060653 |
|
persistent identifier |
https://treatment.plazi.org/id/F966E84E-FF83-2159-FF1A-FD54FBC801AE |
|
treatment provided by |
Felipe |
|
scientific name |
Austroconops annettae Borkent |
| status |
sp. nov. |
Austroconops annettae Borkent View in CoL , new species
Austroconops mcmillani: Borkent, Wirth, and Dyce, 1987 View in CoL . Female adult (in part).
DIAGNOSIS: Male. The only extant Austroconops with flagellomere 12 short, 0.4 the length of flagellomere 13 ( fig. 1B View Fig ). Female. The only extant Austroconops with each claw with a well developed basal tooth ( fig. 1J View Fig ). Egg and larva (all instars). Not distinguishable from those of A. mcmillani (see generic diagnosis above). Pupa. Unknown.
DESCRIPTION: Male adult: Descriptive statistics in table 1. Head: Antenna with well developed plume. Flagellomere 12 elongate, with subbasal constriction, 0.43 length of flagellomere 13 ( fig. 1B View Fig ). Mouthparts moderately long. Palpus ( fig. 1D View Fig ) with 4 segments, segment 3 slightly ovoid in lateral view (more slender than in A. mcmillani ), with capitate sensilla scattered on surface or in shallow pits. Thorax: Scutellum angular in dorsal view. Wing ( fig. 1K View Fig ): Costa extending just beyond apex of R 3. Legs: Legs lacking armature. Bristles on midleg tibia, first tarsomere elongate ( fig. 1N View Fig ). Midleg tibia without apical spur. Hindleg first tarsomere without thick basal spine or stout setae. Claws simple, each claw apically bifid. Genitalia: In life, rotated about 50°. Not distinguishable from that of A. mcmillani . Female adult: Descriptive statistics in table 2. Head: Ommatidia narrowly separated dorsomedially. Antenna as for A. mcmillani . Flagellomeres gradually increasing in length from flagellomere 2 to 13. Mouthparts moderately elon gate, mandible ( fig. 1H View Fig ) narrow, with fine teeth, most directed dorsolaterally, laciniae with well developed, fine retrorse teeth. Palpus ( fig. 1F View Fig ) with 4 segments, segment 3 ovoid, slightly swollen in lateral view, with capitate sensilla scattered on surface or in shallow pits. Thorax: Scutellum angular in dorsal view. Wing ( fig. 1L View Fig ): Costa extending to or just beyond apex of R 3. Legs: Femora, tibiae slender. Legs lacking armature. Midleg tibia without apical spur. Hindleg first tarsomere without thick basal spine or stout setae. Foreleg, midleg, hindleg claws ( fig. 1J View Fig ) nearly straight for apical threefourths, with stout basal tooth. Genitalia: Indistinguishable from that of A. mcmillani . Egg: Descriptive statistics as in table 3. Firstinstar larva: Head capsule length statistics in table 4. Total body length 0.67–0.77 mm (N = 7). Secondinstar larva: Head capsule length statistics in table 4. Total body length 1.43–1.82 mm (N = 4). Thirdinstar larva: Head capsule length statistics in table 4. Total body length 2.05–2.61 mm (N = 8). Fourthinstar larva: Head capsule length statistics in table 4. Total body length uncertain. Pupa: unknown.
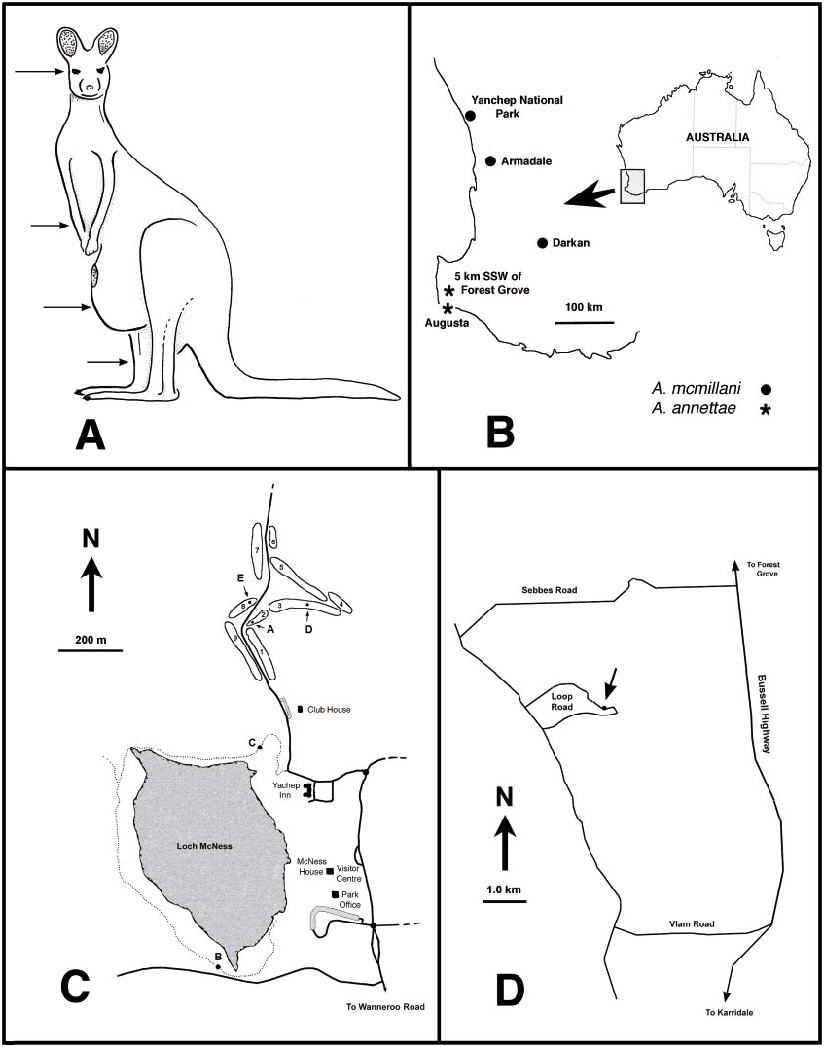
DISTRIBUTION AND BIONOMICS
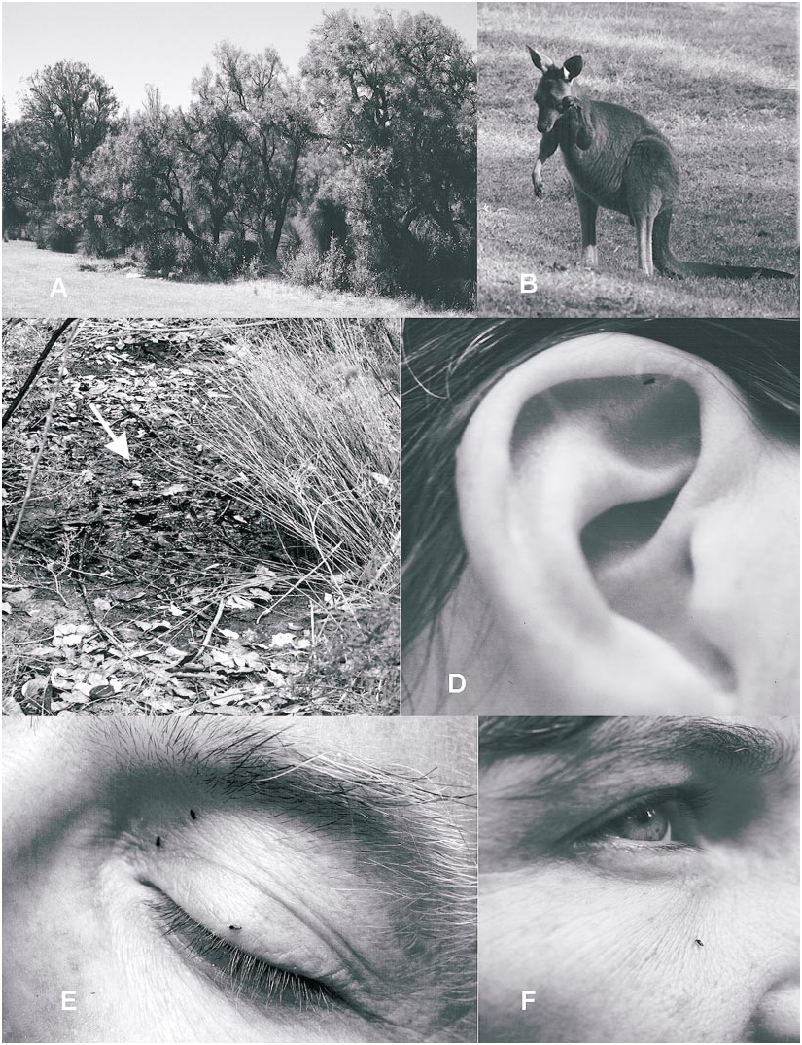
Austroconops annettae is known from two sites in southwestern Australia ( fig. 22B View Fig ). The type locality ( fig. 22D View Fig ) is on Loop Road, 5 km SSW of Forest Grove, WA. One male and four females (one lost after being collected) were swept from a very small patch of low vegetation immediately beside a very shallow (less than 3 cm deep) small pool less than a meter in diameter ( fig. 21C View Fig ). The pool was immediately beside and south of the narrow track of Loop Road and was located in a dry, shallow streambed (likely with running water during the winter). Surrounding vegetation was composed of an open Jarrah– Marri forest with thick stands of shrubs ( Leucopogon parviflorus was common).
The single female from Augusta ( fig. 22B View Fig ) was collected with a sweepnet and was either collected 1.6 km south of Augusta (most likely) or about 1.6 km east of Jewel Cave (about 7.5 km NW of Augusta) (D. Colless, personal commun.).
Two females found on November 2, 2001 at the type locality were collected between 3: 00 and 4:00 PM when the ambient temperature was 20°C (but substantially warmer in the sun). When the single female retained live in a vial was at 17°C, she became very lethargic (nearly torpid), but when the vial was warmed up, she again became very active. A third female collected on November 4 was lost but was collected at 12:50 PM and at 22°C. One male was collected on November 13 at 1:15 PM at 33°C, and one female was found on the same date and temperature at 3:30 PM. Fervent and repeated sweeping all around this immediate site on each of the above dates failed to produce any further specimens, which suggests that the few adults were indeed concentrated at the small pool that appeared to be the only open water (although very small) in a large area. Samples of mud from the edge and bottom of this small pool failed to produce any immatures.
The distinctive claw of female A. annettae ( fig. 1J View Fig ), with a pronounced inner basal tooth moreorless situated in the same plane as the rest of the claw, may indicate the type of host upon which the female feeds. Within the Simuliidae , females of species with a similarly shaped claw are restricted to those which feed on birds ( Crosskey, 1990). Within the Ceratopogonidae , the only species of vertebrate feeders with variation regarding the presence or absence of an inner basal tooth are those in the genus Leptoconops ( Forcipomyia Meigen (Lasiohelea Kieffer) and Culicoides all have simple claws with at most, a small, very slender spicule). Nearly all Leptoconops which bite mammals have a simple claw or a claw with a small basal spicule. Species with a claw with a pronounced inner basal tooth feed either on humans (e.g., L. spinosifrons (Carter) and L. siamensis Carter ; Chanthawanich and Delfinado, 1967) or on birds ( L. werneri Wirth and Atchley ; Wirth and Atchley, 1973), indicating that the shape of the claw may not strictly indicate host type in Leptoconops . Other species which have a claw shape virtually identical to A. annettae are L. freeborni Wirth , L. melanderi Wirth and Atchley and L. patagoniensis Ronderos , but their hosts are unknown. Further research is warranted because we do not have host records for many species of Leptoconops . Lane
TABLE 6 Data on Rearing of Austroconops annettae (2001–2002)
(1977) has shown that the claw shape of females of species of Forcipomyia ( Trichohelea Goetghebuer ) are adaptations to cling to the scales of butterfly wings as females feed on butterfly blood (they pierce the wing veins to obtain this). If further study shows a relationship between the claw shape of females of Austroconops and Leptoconops and vertebrate host type, it will provide the basis for interpreting such variation in the fossil record: Lebanese amber species Austroconops gladius and A. gondwanicus , 121 million years old, both have large basal teeth on their claws similar to those of A. annettae ( Borkent, 2000a) .
The female allotype collected on November 13 laid eggs scattered separately on the surface of wet mud in a vial or on the sides of the vial just above the mud. The sequence of egg laying, hatching, and larval development is given in table 6. Larval behavior was indistinguishable from that of A. mcmillani (see above). In addition to the feeding observations associated with the fecal infusion described under A. mcmillani , one firstinstar larva A. annettae was observed to eat a small live nematode whole (in less than 2 seconds). A second larva, upon encountering a somewhat moribund large nematode, pierced it at midlength. This was followed by rapid movement of the pharyngeal complex and ingestion of part of the nematode; it did not complete feeding on the nematode. In spite of these observations, the first to fourthinstar larvae mostly ignored the large number of nematodes added to the Petri dishes every 2–3 days and, similar to larvae of A. mcmil lani, congregated near fresh drops of the fecal infusion, suggesting that they too primarily feed on microorganisms.
TAXONOMIC DISCUSSION
Austroconops annettae is very similar to A. mcmillani in all its stages (pupa not known) and we were unable to distinguish differences in male or female genitalia, eggs, or the different larval instars (except for some size differences in the immatures, which may have been due to restricted sample size or laboratory rearing conditions; tables 3, 4).
The firstinstar larvae are described as having nine undivided segments ( fig. 2C View Fig ). However, in A. mcmillani , early firstinstar larvae also appear to have undivided segments, whereas older firstinstar larvae have divided segments. The same is likely true for A. annettae .
The female from Augusta was included as a specimen of A. mcmillani View in CoL in the analysis by Borkent et al. (1987).
TYPE MATERIAL
Holotype, male adult on microscope slide, labeled ‘‘ HOLOTYPE Austroconops annettae Borkent , 5 km SSW of Forest Grove, Loop Road , WA, Australia, 13XI2001, A. Borkent, CD1998’’ ( WAMP); allotype, female adult on microscope slide, labeled as for holotype and ‘‘Female which laid eggs, resulting in 1–4 instar larvae’’ ( WAMP); paratypes 3 females, 7 eggshells, 8 firstinstar larvae, 6 secondinstar larvae, 9 thirdinstar larvae, 1 fourthinstar larva, 1 terminal portion of abdomen of second or thirdinstar larva, all reared from eggs laid by female allotype; 2 females from type locality but collected 2XI2001 ( CNCI); 1 female from Augusta, WA, 3X1970 ( ANIC); immatures ( CNCI) .
In addition to the above material, more eggs, eggshells, and specimens of the different larval instars were studied but were either left to develop further or were subsequently lost. Therefore, the numbers in tables 3 and 4 that record specimens do not match that listed above.
DERIVATION OF SPECIFIC EPITHET
This species is named after the first author’s wife, Annette Borkent. She shared in virtually every aspect of the sixweek expedition to study Austroconops , and the results of this paper would not have been possible without her continuous support.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Austroconops annettae Borkent
| BORKENT, ART & CRAIG, DOUGLAS A. 2004 |
Austroconops mcmillani
| : Borkent, Wirth, and Dyce 1987 |
A. mcmillani
| : Borkent, Wirth, and Dyce 1987 |