Coptotriche carmencita Stonis & Diškus, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4691.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:14CC7B3E-ACBB-4770-A9D2-3AD35A1A2532 |
|
DOI |
https://doi.org/10.5281/zenodo.5930151 |
|
persistent identifier |
https://treatment.plazi.org/id/679162F7-9DD9-4767-80E2-CF0AD29F5A88 |
|
taxon LSID |
lsid:zoobank.org:act:679162F7-9DD9-4767-80E2-CF0AD29F5A88 |
|
treatment provided by |
Plazi |
|
scientific name |
Coptotriche carmencita Stonis & Diškus |
| status |
sp. nov. |
Coptotriche carmencita Stonis & Diškus View in CoL , sp. nov.
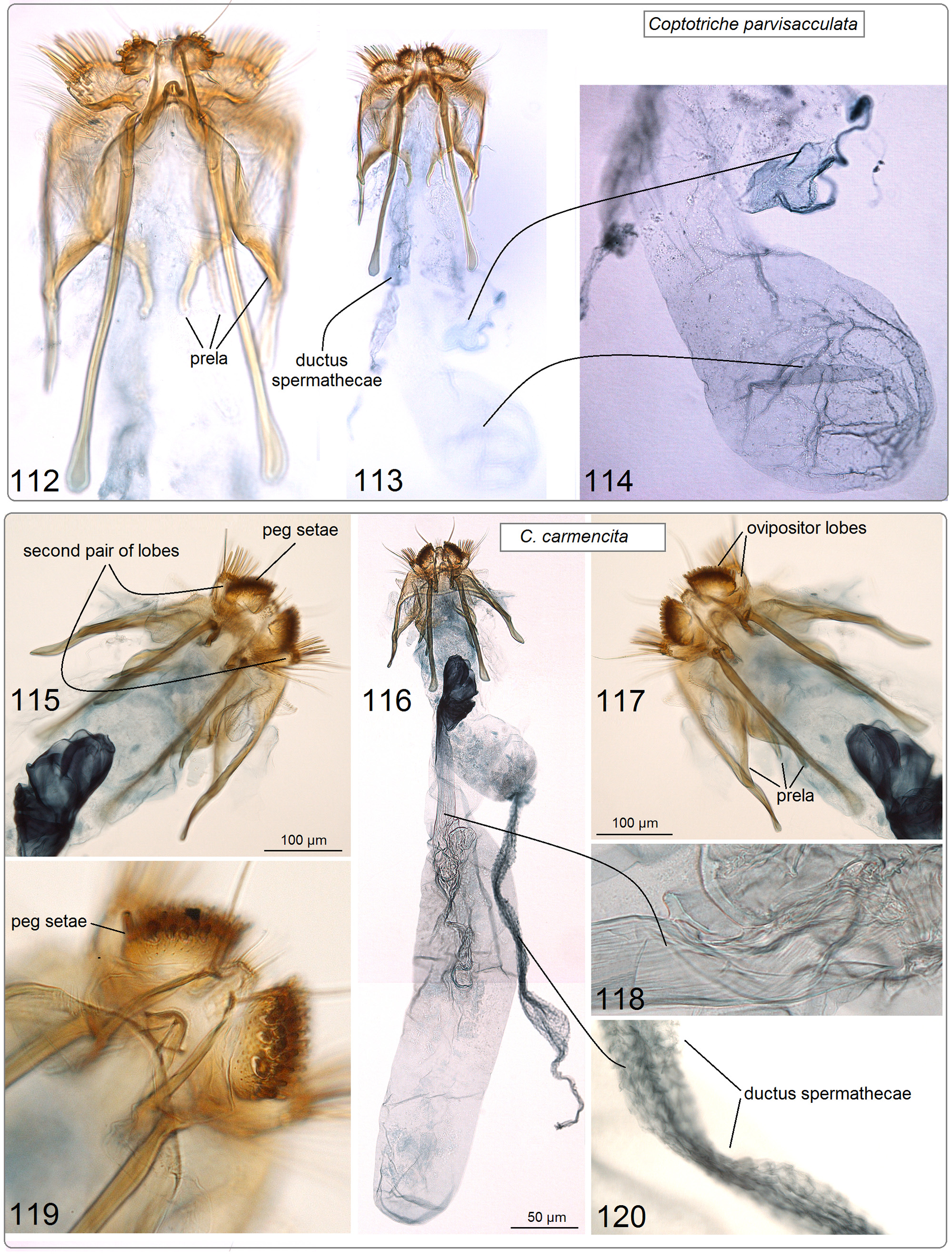
( Figs 23, 24 View FIGURES 19–24 , 57–62 View FIGURES 57–62 , 115–120 View FIGURES 112–120 )
Type material. Holotype: ♂, PERÚ: Junín Region, La Merced, 11 ° 04ꞌ02.7ꞌꞌS, 75 ° 20ꞌ37.7ꞌꞌW, Fundo San José , 840–900 m, at light, 8–17.v.2018, leg. J. R. Stonis & S. R. Hill, with participation of J. Puplesyte-Chambers, genitalia slide no. AD992 ( ZMUC) . Paratypes: 1 ♂ (with abdomen missing), 1 ♀, same label as holotype, genitalia slide no. AD 993♀ ( ZMUC) .
Diagnosis. External characters are not sufficient for species identification. In the male genitalia, the combination of a short, slender, triangular vinculum ( Fig. 61 View FIGURES 57–62 ), weakly developed spines on the phallus ( Fig. 62 View FIGURES 57–62 ), strongly spined diaphragma ( Fig. 61 View FIGURES 57–62 ), and a membranous, wrinkled anellus ( Fig. 59 View FIGURES 57–62 ) distinguishes C. carmencita from all known Coptotriche species. In the female genitalia, the combination of a bulbous accessory sac ( Fig. 116 View FIGURES 112–120 ), very large and elaborated ductus spermathecae ( Fig. 120 View FIGURES 112–120 ), and long corpus bursae ( Fig. 116 View FIGURES 112–120 ) distinguishes the new species from all known congeneric species.
Description. Male ( Figs 23, 24 View FIGURES 19–24 ). Forewing length 2.6–2.7 mm; wingspan 5.6–5.8 mm (n = 2).
Head. Face, labial palpus; frontal tuft, and collar concolorous, yellow cream to yellow-ochre; antenna distinctly longer than one-half length of forewing; flagellum glossy, greyish cream to yellow cream.
Thorax. Tegula, thorax, and forewing concolorous, glossy, ochre-yellow, sparsely speckled with grey scales, and with some purple iridescence, particularly distinct along forewing dorsum; fringe grey, with incomplete and inconspicuous fringe line, comprised of black scales; forewing underside densely covered with pale grey to dark grey scales, with slight purple iridescence, without spots or androconia. Hindwing glossy white to pale grey (depending on angle of view), without androconia; fringe ochre cream to pale grey. Legs very glossy, cream, with some grey and black grey scales on upper side.
Abdomen. Grey cream, with distinct metallic gloss on upper side, pale grey, distally yellow-ochre or whitish cream on underside; anal tufts inconspicuous, yellowish cream. Genitalia ( Figs 57–62 View FIGURES 57–62 ) with capsule about 365 µm long. Uncus with two very large lateral lobes ( Figs 58, 60 View FIGURES 57–62 ). Valva broad, undivided ( Figs 58, 61 View FIGURES 57–62 ), about 275 µm long (excluding the basal process); transtilla short ( Fig. 57 View FIGURES 57–62 ); basal process of valva short ( Fig. 58 View FIGURES 57–62 ). Anellus membranous, wrinkled ( Fig. 59 View FIGURES 57–62 ). Diaphragma spined ( Fig. 61 View FIGURES 57–62 ). Phallus about 515 µm long, strongly broadened in distal third, with weakly developed spines ( Fig. 62 View FIGURES 57–62 ).
Female. Externally similar to male.
Genitalia ( Figs 115–120 View FIGURES 112–120 ) about 1800 µm long. Ovipositor lobes large ( Fig. 117 View FIGURES 112–120 ) with short, stout and darker, modified setae (‘peg setae’) ( Fig. 119 View FIGURES 112–120 ); area between ovipositor lobes indistinct, with tiny papillae and some short setae. Second pair of lobes, lateral and anterior to ovipositor lobes, much smaller than ovipositor lobes, but bearing very long slender setae, without stout, modified peg setae. Posterior apophyses longer than anterior ones ( Figs. 115, 116 View FIGURES 112–120 ); prela comprised of three pairs of projections ( Fig. 117 View FIGURES 112–120 ). Corpus bursae long and narrow ( Fig. 116 View FIGURES 112–120 ), without pectinations or signum. Accessory sac bulbous ( Fig. 116 View FIGURES 112–120 ); ductus spermathaecae very large, elaborate, but without coils ( Figs 116, 120 View FIGURES 112–120 ); utriculus absent or not preserved.
Bionomics. The host plant is unknown. Adults occur in May, and fly to light.
Distribution. The species is known from the single locality, La Merced, Junín Region, central Peru, at an elevation of about 900 m, from the “selva alta” ( Figs 19–21 View FIGURES 19–24 ).
Etymology. The species name “carmencita” is a diminutive version of the feminine name Carmen. The name (instead of the correct Latin version “carmencitae”) was chosen as an arbitrary combination of letters and in honor of señora Carmen Brocq Tremolada, who realised her dream to create the Ecological Park Fundo San José and open it to the public and researchers. Jonas R. Stonis and Julia Puplesyte-Chambers are indebted to señora Carmen for her friendly welcome during our studies at Fundo San José.
| ZMUC |
Zoological Museum, University of Copenhagen |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.