Dushia wijnhoffae Schwartz & Norenburg, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4691.4.2 |
|
publication LSID |
lsid:zoobank.org:pub:875FB4E7-148A-4AC2-904C-174416B11256 |
|
persistent identifier |
https://treatment.plazi.org/id/03B387A4-FFF7-CC29-FF73-C4D9FAE8FDE5 |
|
treatment provided by |
Plazi |
|
scientific name |
Dushia wijnhoffae Schwartz & Norenburg |
| status |
sp. nov. |
Dushia wijnhoffae Schwartz & Norenburg sp. nov.
( Figs 1A View FIGURE 1 , 2 View FIGURE 2 A–E, 3A–D)
Dushia atra: Corrêa 1963, p. 44 –48.
? Cerebratulus ater: Stiasny-Wijnhoff 1925, p. 108 –109.
? Lineus ater: Coe 1951a, p. 181 ; 1951b, p. 330.
Not Meckelia atra Girard, 1851, p. 291 ; 1893, p. 163.
Not Cerebratulus ater: Verrill 1895, p. 531 ; Bürger 1904, p. 124; Coe 1943, p. 253.
Etymology. The specific epithet wijnhoffae is the latinized form of Gerarda Stiasny-Wijnhoff’s maiden name, chosen by us to link her with the author of Dushia, Diva Corrêa , another of the great pioneers in nemertean systematics.
Material examined. Holotype: USNM 1594812 View Materials , serial frontal sections of anterior to foregut (14 slides), collected from Anse de Grand Fond , St. Barthélemy, France from under stones on coarse shell hash and sand just at the water line in August 2003 . Paratypes: USNM 1594813 View Materials , serial transverse sections of anterior, foregut, mid-body, and intestinal regions (99 slides) Corrêa’s (1963) voucher slides and specimens are presumed lost (her entire collection known to have been in her laboratory has been missing for many years) .
Additional material. Six whole specimens fixed in 10% seawater-buffered formalin and preserved in 70% EtOH, and four samples preserved in 95% EtOH were collected from Bahia de la Chiva and Blue Beach, Vieques, Puerto Rico in June 2007 and August 2011; two of those DNA extracts are available at the USNM as JLN0657 _ 0611_001 and 002. Sixteen specimens collected in Saint Barthélemy in 2002, 2003 and 2007 were preserved in 95% ETOH and extracted for DNA. Those genomic extracts are available at the USNM as JLN0657 _ 030502 _ 001, 002, 008, 010, 011, 012, 013, 014; JLN0657 _ 030803 _ 003, 004; JLN0657 _0301103_004; JLN0657 _ 031207 _001, 002, 003, 004, 005.
Coe (1951a, b) identified several specimens as Lineus ater that were deposited in the Smithsonian Institution’s National Museum of Natural History and include the following: USNM 1263, collected in February 1884 from Curaçao by the Steamer Albatross R/V; USNM 22085, collected by Henry Hemphill in 1885 from the Florida Keys at low tide; and USNM 24750, collected in Louisiana from under rocks.
Diagnosis. Heteronemertean with black body and dorso-ventraly white-tipped head; head shape retuse; ocelli absent; anterior foregut region moderately rounded becoming moderately dorsoventrally flattened but without sharp lateral margins present.
Description. External features. The material examined agrees with Corrêa’s (1963) description of D. atra , except for her conclusion that a caudal cirrus is lacking. The cirrus is tiny and its presence is, in fact, extremely difficult to ascertain but can be observed in living animals as a discrete entity. Additional details are from examination of histological sections and observations of live specimens.
The foregut region is slightly rounded in living specimens. The intestinal region is not distinctly flattened ( Fig. 1A View FIGURE 1 ), and lacks sharp lateral margins or any internal dorsoventral musculature (dvm) external to the lateral nerve cords (lnc) that normally is associated with swimming cerebratulids. Girard (1851), Coe (1943), and Corrêa (1963) indicate that their specimens were between 15–50 cm long, whereas our samples are between 5 and 15 cm. When living specimens are handled, they are sticky to touch. The worms have not been observed to swim even after prolonged nudging with a dissecting needle.
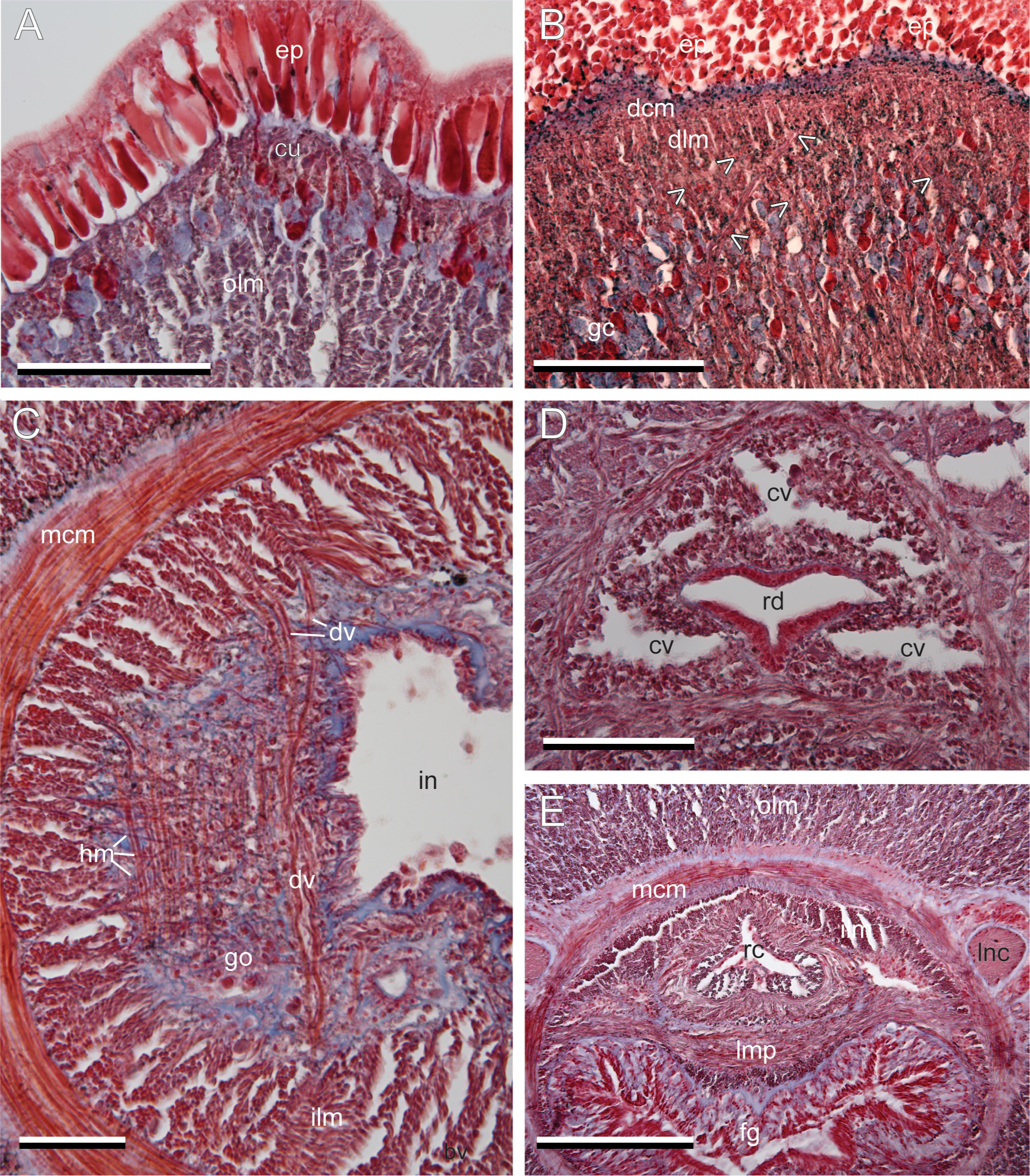
Body wall and musculature. The epidermis contains numerous red and yellow staining goblet-shaped cells and ciliated cells that rest on a distinct basal lamina ( Fig. 2A View FIGURE 2 ). A thin layer of dermal musculature is present below the basal lamina of the epidermis. It includes a circular muscle layer only 2–3 fibers thick, a lattice-like arrangement of diagonal muscle fibers, and longitudinal muscle layer approximately 2–5 fibers thick ( Fig. 2A, B View FIGURE 2 ). Mucoid and serous gland cells are present in the cutis and define its border with the olm. Pigment granules are extracellular and are distributed in the epidermis and cutis.
There are a few sparsely distributed mucoid gland cells present at the anterior most portions of the head near the apical organ pits. Acidophilic gland cells are located in a distinct arrangement as in a row across the ventral surface and dorsolaterally in two groups on either side of the rhynchodeal complex, just internal to the epidermis beginning in the anterior of the head and extending posterior to the cerebral ganglia region.
The remaining body wall is typical, three-layer heteronemertean construction. Radial muscles run between the epithelium of the foregut and the epidermal basal lamina. The foregut and intestine are lined by a glandular epithelium with a few sparsely distributed muscle fibers.A plate of longitudinal muscles is present between the rhynchocoel musculature and the foregut epithelium in the region of the mouth ( Fig. 2E View FIGURE 2 ). The rhynchocoel musculature is not interwoven with the body wall muscles.
Proboscis apparatus. Corrêa (1963) described the proboscis is as three layered with (terminology from everted form) a glandular epithelium, outer longitudinal muscle layer, a neural plexus, an inner circular and longitudinal layer, and two muscle crosses. Corrêa’s (1963) description does not indicate if pseudocnidae-like structures are present in the epithelium, and we did not observe them in our material.
Alimentary canal. Intestinal diverticula can be identified by the presence of dorsoventral (dv) musculature that insert in the dorsal and ventral portions of the middle circular muscle (mcm) fibers passing through the inner longitudinal muscle (ilm) layer on either side of the intestine ( Fig. 2C View FIGURE 2 ). Most of the dv musculature is external to the lateral blood vessels in the intestinal region with a few fibers passing internally to the vessel. The diverticula are also associated with a layer of longitudinally oriented muscles that are overlain by the dvm fibers and are held together by connective tissue.
Circulatory system. Corrêa (1963) identified four cephalic vessels in the circulatory system; a larger pair arranged laterally to the rhynchodaeum and a smaller pair arranged dorsal and laterally to the rhynchodaeum. The two smaller vessels have perforations in the septum separating them. In our material, the two small vessels are arranged as a single dorsal vessel for most of its length but does contain several partitions ( Fig. 2D View FIGURE 2 ). Corrêa (1963) also did not observe a rhynchocoel vessel or villus. In our material, a vessel is present along the ventral portion of the rhynchocoel from the mouth and foregut regions to the intestinal region where it exits from the rhynchocoel ventrally and becomes the intestinal dorsal vessel positioned between the intestine and rhynchocoel.
Excretory system. The specimen sectioned was regenerating parts of foregut regions including the proboscis and rhynchocoel, near to the region where the nephridial system is usually found. The excretory system begins as a series of branching tubules in the region of the mouth. From our sample it is not clear if these branches are connected to the tubules in the foregut, although Corrêa (1963) indicates that they are a single continuous system. The nerphridial ducts lead away from the tubules dorsolaterally to nephridial pores.
Nervous system and sense organs. Bürger type I, II, III and neurocord cells were observed in both the dorsal and ventral ganglia and the anterior most portions of the lateral nerve cord (lnc) ( Fig. 3A, B View FIGURE 3 ). A few neurocords can be identified within the fibrous part of the lateral nerve cords, but only in the region near to the ganglia.
A distinctive U-shaped ridge of cells with long cilia and interspersed gland cells is present in the cephalic furrows flanking the entrance to the cerebral organ ( Fig. 3C, D View FIGURE 3 ). This ridge can be observed in living, relaxed specimens by separating the cephalic furrows with needles. This feature is not usually noted in descriptions of heteronemerteans, yet is present in all heteronemerteans examined except those without cephalic furrows (MLS pers. obs., Figs 3C, D View FIGURE 3 , 6D View FIGURE 6 ). Riser (1990) was the first to notice the structure, and named the genus Adenorhagas after it, however, it is not a structure restricted to this genus.
Reproductive system. The material examined was spawned out and the gonads appeared to be in the process of regressing. The gonads are arranged serially between the intestinal diverticula walls. Gonoducts and gonopores were not observed.
Habitat and distribution. Dushia wijnhoffae sp. nov. is littoral, found under stones partially embedded in course shell hash and sand at the water line. Their particular habitat is very clean without much additional organic material and has few other inhabitants. They are easy to identify in the field since their black pigmentation makes the worms conspicuous against the white and brown of the shell and sand when turning rocks. Specimens have been collected or observed from the following locations in the Caribbean: St. Barthélemy, France ( JLN), Bahia de la Chiva and Blue Beach, Vieques, Puerto Rico, USA ( MLS), Dominica (Leslie Harris, pers. comm.), and Curaçao ( Stiasny-Wijnhoff 1925; Corrêa 1963). Material deposited at NMNH implies that they are also found in littoral habitats in the Gulf of Mexico and in the Florida Keys.
Remarks. Meckelia atra Girard, 1851 was originally described from a single specimen dredged in deep waters off Cape Florida ( Girard 1851, 1893). Girard’s (1851) description is short and indicates that the specimen had a flattened body, was black throughout except with a paler anterior, and was 15 cm long after preservation. Later, Verrill (1895) made Meckelia a junior synonym to Cerebratulus . Coe (1951a) eventually transferred it to Lineus , presumably because of the lack of a caudal cirrus. It is not clear if Coe (1951a) examined any living samples and he may have been working with material from the Steamer Albatross collected in 1884 and 1885 that is deposited in the NMNH collection.
Stiasny-Wijnhoff (1925) collected two headless specimens littorally in “Spanish waters” off Curaçao but was unsure that they were the same as Girard’s (1851) material and placed a “?” mark in her description of the fragments. Stiasny-Wijnhoff (1925) apparently dissected the animals or cut histological sections because she noted the presence of distinct connective tissue layer between the cutis and olm layers in her specimens. Corrêa (1963: 51) col- lected in Curaçao and returned to Stiasny-Wijnhoff’s (1925) sites but was not able to find any specimens and stated that the habitat was not correct for this particular species. In her histological examination of the Curaçao material, Corrêa (1963) found that they did not possess a connective tissue layer as Stiasny-Wijnhoff (1925) observed. The material examined here does not possess a thickened connective tissue layer, it is of minimal thickness, and mostly associated with the proximal outer longitudinal muscle fibers rather than a discrete layer ( Fig. 2A, B View FIGURE 2 ). Whether Girard’s (1851, 1893) and Stiasny-Wijnhoff’s (1925) specimens are conspecific or not remains indecisive, but these are apparently different from Corrêa’s (1963) and ours, and the latter two undoubtedly represent the same species.
In St. Barthélemy, despite extensive sampling of all sand habitats around the island, the worms were found exclusively at the west end of Grand Fond in the intertidal wash zone behind a fossil reef that is exposed at low tide. They are abundant, often in clusters of two or three but as many as 10 or more worms, under large rubble lying on coarse carbonate (coral) sand; the worms burrowed very rapidly into this sand when exposed. Associated fauna includes shelled micro-mollusks, small crustaceans and members of three species of Ototyphlonemertes . In captivity the worms enthusiastically react to the scent of and feed on several kinds of crustacean, mollusk and fish tissues but, despite a very large mouth, the tissues must be chopped into cubes of 2 mm or less; these are swallowed whole.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Dushia wijnhoffae Schwartz & Norenburg
| Hookabe, Natsumi, Schwartz, Megan L., Kajihara, Hiroshi & Norenburg, Jon L. 2019 |
Dushia atra: Corrêa 1963 , p. 44
| Correa 1963: 44 |
Lineus ater
| : Coe 1951: 181 |
Cerebratulus ater:
| Stiasny-Wijnhoff 1925: 108 |
Cerebratulus ater:
| Verrill 1895: 531 |
Meckelia atra
| Girard 1851: 291 |