Euphanerops longaevus, Woodward, 1900
|
publication ID |
https://doi.org/ 10.5281/zenodo.5377455 |
|
persistent identifier |
https://treatment.plazi.org/id/038C87B0-BB57-FF88-FD02-FC25FC4422CD |
|
treatment provided by |
Marcus |
|
scientific name |
Euphanerops longaevus |
| status |
|
RECONSTRUCTION OF EUPHANEROPS LONGAEVUS
The reconstruction of Euphanerops longaevus raises a number of questions, partly because of its unusual morphology (e.g., a very large number of gill arches), but chiefly because of the uncertainty as to the way the specimens have collapsed during decay, and the biogenic or diagenetic nature of the mineralized structures we observe in the large specimens.
E. longaevus can be readily compared to anaspids, with which it shares a markedly hypocercal tail and elongated, ventrolaterally placed paired fins, although the latter are only known from their dermal skeletal covering in anaspids. Conversely, E. longaevus shows no unambiguous element of the dermal skeleton that would allow more detailed comparison with the anaspids (except perhaps for the presumed elongated lateral scales). Comparisons with other major vertebrate taxa for which there is good information about the internal anatomy, namely hagfishes, lampreys, galeaspids, osteostracans, placoderms, and crown-group gnathostomes, leaves us with very few uniquely shared characters, except perhaps when considering lampreys, with which E. longaevus shares sinuous gill arches forming a “branchial basket”. By its very elongated branchial apparatus, E. longaevus also closely resembles other Devonian soft-bodied jawless vertebrates, namely Endeiolepis , Achanarella , Cornovichthys , and possibly Jamoytius ( Stensiö 1939; Ritchie 1984; Arsenault & Janvier 1991; Janvier 1996 a; Newman & Trewin 2001; Newman 2002; Janvier et al. 2006; see below). Certain galeaspids have a very large number of gills (up to about 45), but, contrary to the condition in E. longaevus , they never reach the anal region, which, by comparison to the anatomy of osteostracans, can be located further back, where the series of ventrolateral ridge scales meet along the midline (Janvier 2004).
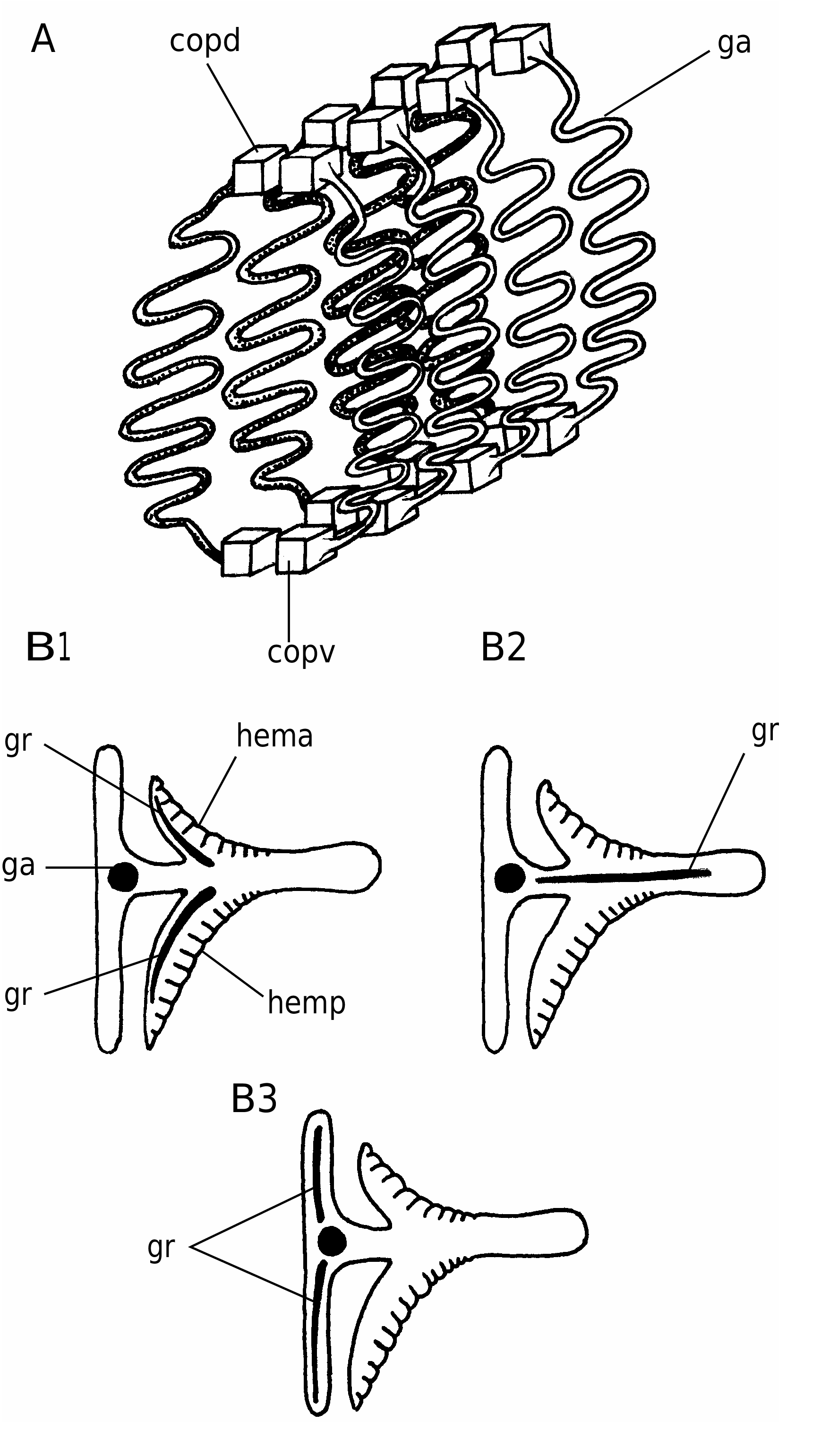
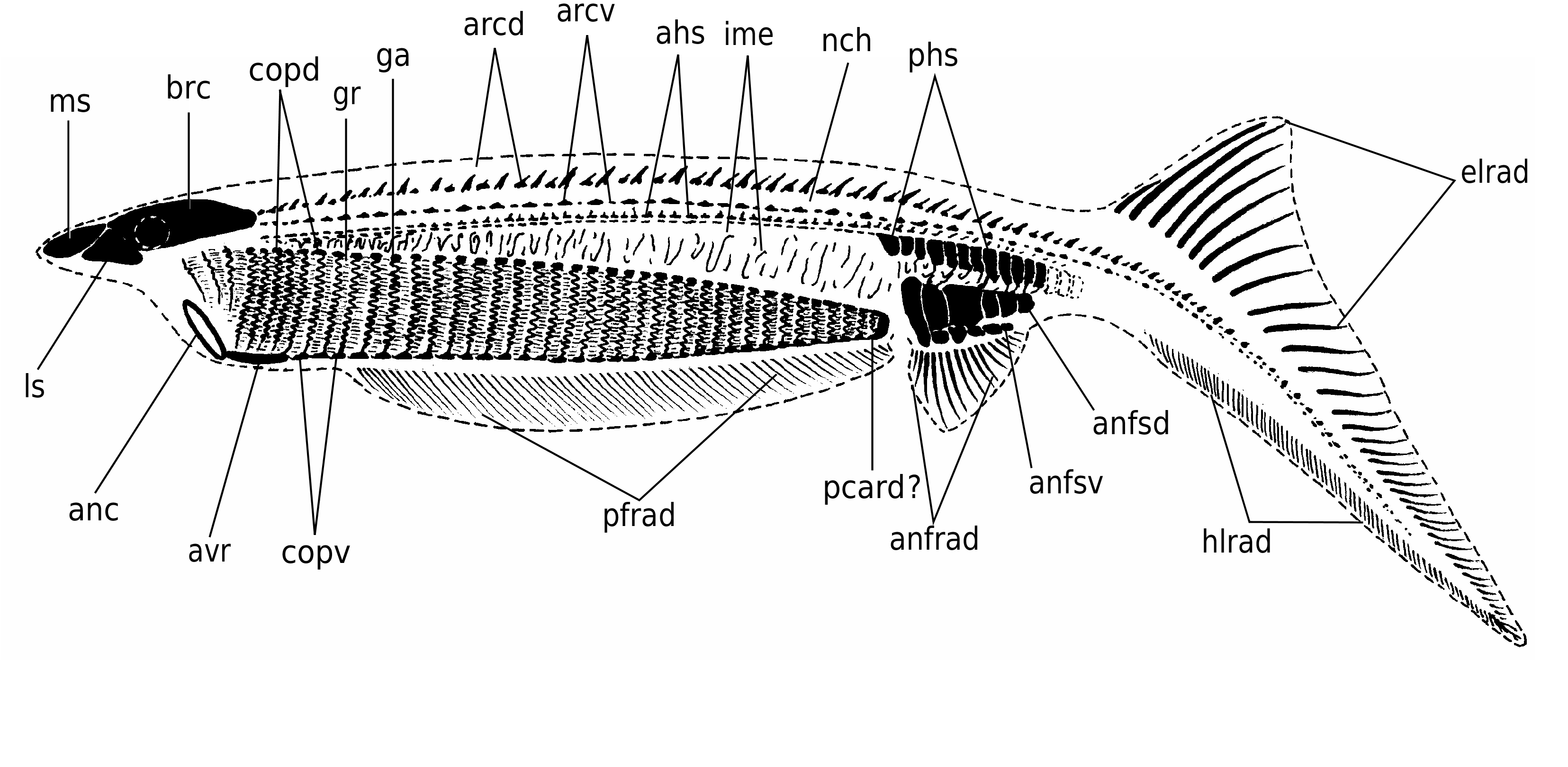
In detail, the reconstruction of the branchial apparatus of E. longaevus remains difficult. Here, we have assumed that the gill arches were united dorsally and ventrally to a paired series of relatively large copular elements (ga, copd, copv, Figs 26A View FIG ; 40 View FIG ). As for the “gill rods” (gr, Fig. 26B View FIG ), new data on the three-dimensionally preserved branchial apparatus of Endeiolepis aneri provide information that enlighten the structure of E. longaevus , all the more so that the two taxa are probably synonyms ( Janvier et al. 2006). The “gill rods” were possibly situated in the interbranchial septa that separated adjacent gill pouches or supported the anterior and posterior hemibranchs of the same arch. Morever, the fact that the “gill rods” of E. longaevus are generally not spread around the body suggests that they were enclosed in the branchial apparatus and did not extend into gill covers, as in chondrichthyans.
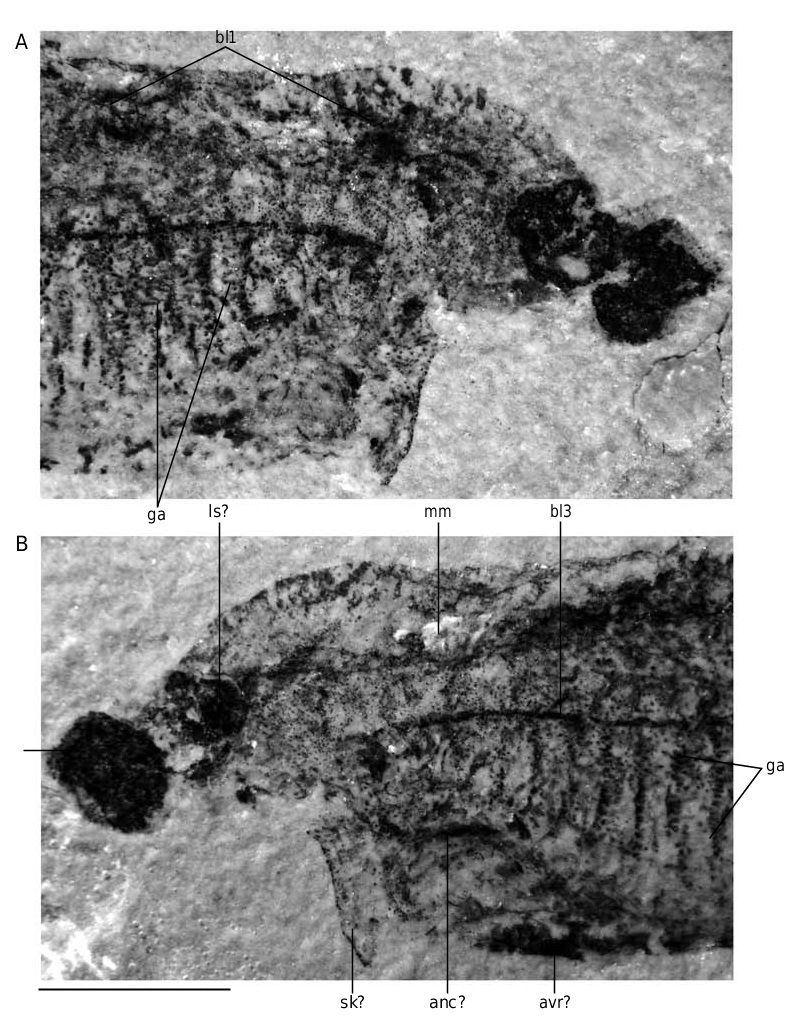
The position and the role of the “annular cartilage” remains uncertain. In all specimens where it is preserved, it lies at the entrance of the branchial apparatus, and seems more or less obliquely oriented (anc, Fig. 40 View FIG ). One may imagine that it served in maintaining open the incurrent opening of the branchial apparatus, or surrounded the oral opening. Although such a ring-shaped cartilage is only known elsewhere in lampreys, in the form of the annular cartilage, the position of the latter is different, and much more anteriorly placed. There is thus no certainty about the homology of these two ring-shaped structures.
The “braincase” and snout (possibly strengthened by cartilaginous plates, if the “head stains” actually are cartilages) were slightly overhanging the presumed incurrent opening at the anterior limit of the branchial apparatus, as suggested by the aspect of most laterally collapsed specimens (e.g., Figs 3 View FIG ; 4 View FIG ; 18 View FIG ; 40 View FIG ), but the main question raised by this peculiar anatomy is that of the position and organization of the mouth and oral cavity. The anatomy of the branchial apparatus is suggestive of particulate suspension feeding and ram-ventilation ( Mallatt 1984). Such a mode of life in a jawless vertebrate is not unlikely, but would imply that the oral cavity was continued posteriorly by the pharynx, much as in larval lampreys and living gnathostomes. Assuming that the mineralization of the skeleton is biogenic, this would perhaps explain the fact that the gill arches could become extensively mineralized and thus partly lose their flexibility, without posing any functional problem. However, the new data provided by Endeiolepis aneri , which displays exactly the same type of elongated branchial apparatus as E. longaevus , suggest that the gills were enclosed in numerous, crowded pouches ( Janvier et al. 2006). This, in turn, implies that respiration was effected through passive inspiration, as in lampreys ( Mallatt 1996), and thus that the gill arches retained some flexibility throughout life.
There is unfortunately no indication as to the anatomical relationships between the mouth and the pharynx, and between the posterior end of the pharynx and the digestive tract. All we know is that the latter comprised a relatively large stomach filled with very fine-grained sediment. The anterior limit of the branchial apparatus, seems to coincide with the anteroventral limit of the head. The “annular cartilage” was also probably situated at this level ( Fig. 40 View FIG ).
Inferences based on lamprey anatomy must take into consideration two possible models: the larval condition, in which the oral cavity, the pharynx (branchial apparatus) and the digestive tract are in direct continuity, and the adult model, in which the branchial apparatus is a cul-de-sac, connected to the oral cavity by an oesophagobranchial duct, and thus does not convey the food particles to the digestive tract. The larval lamprey model would be consistent with what we can infer from the structure of the pharynx in E. longaevus ; that is, the mouth would be a large opening, more or less posteroventral to the “head stains”, possibly strengthened by the “annular cartilage”, and continued posteriorly by a very elongated pharynx that extends back to the anal region. However, this model implies that the pharynx is, in turn, continued posteriorly by the oesophagus, stomach and intestine, which, based on the imprints of the visceral cavity, must have lain dorsally to the branchial apparatus. Therefore, assuming that the food passed through the entire pharynx (or was filtered by the pharynx) implies that the oesophagus formed an anterodorsally directed loop to meet the stomach, and that the posterior intestine formed a posterodorsally (or posterolaterally) directed loop to reach the anus ( Fig. 41A View FIG ). Such a condition in unknown in living jawless vertebrates, but is frequently met with in a wide range of living jawed vertebrates, notably chondrichthyans, where the loops of the posterior intestine are generally either lateral or ventral to the stomach ( Pernkopf & Lehner 1937). The adult lamprey model would be more consistent with the posterior extension of the branchial apparatus. The food would enter the oral cavity, situated somewhere beneath the level of the “head stains” or behind the “annular cartilage”, and then conveyed to the stomach by the oesophagus that passed dorsally to the branchial apparatus ( Fig. 41B View FIG ). The intake of the respiratory water could have been effected through the mouth and oral cavity, but then conveyed to the branchial apparatus by a median pharyngobranchial duct extending between the paired series of gill pouches, and connected to the latter by a series of individual branchial ducts (or branchial pores). This reconstruction is admittedly more consistent with the unusual size of the branchial apparatus and dorsal position of the stomach in E. longaevus , but also raises some questions. The fact that we cannot see any imprint of what could be an oesophagobranchial duct is perhaps not surprising, but the fact that the anterior limit the branchial apparatus coincides with the presumed position of the mouth leaves little space for such a duct. In addition, the adult lamprey model entails an active mode of feeding, thus a relatively complex feeding apparatus, for which we have no evidence in E. longaevus . The hagfish model would resolve the problem of the food transmission through the pharynx, assuming that the mouth served both the water and food intake (there is no indication of a separate nasopharyngeal duct of hagfish type), but the question of the position and path of the oesophagus, posteriorly to the branchial apparatus, would remain the same and inevitably entail a loop of the digestive tract.
There is no clear indication of the position of the eyes, unless the “lateral stains” or the “doughnutshaped structures” are actually collapsed scleral capsules. If such is the case, assuming that the elongation of the snout is exagerated by the dorsoventral flattening of the carcasses and that the “head stains” have lain in a more frontal position, the overhanging snout and almost frontally placed eyes of E. longaevus , bear some resemblance to some other fossil vertebrates, which display various types of rostralization, notably euconodonts and arandaspids ( Gagnier 1993a; Donoghue et al. 2000).
Although the aspect of the “head stains” is similar to that of the three stains described in other softbodied jawless vertebrates (notably Achanarella and Jamoytius ) and classically regarded as imprints of the median olfactory organ (or the annular cartilage) and the eyes, the presence of mineralized matter in the “head stains” of the large specimens of E. longaevus raises questions about this interpretation, at any rate if this mineralization is either pre-mortem, or, if a substrate microfabric preserves the actual structure of the cartilage. If these “head stains” are in fact cartilages, the “median stain” somewhat compares in position and shape to the large, posterior median tectal cartilage of adult lampreys (ms, Fig. 40 View FIG ). In contrast, the “lateral stains” do not compare to any of the cartilages in the adult lamprey snout, except possibly the small paired spinose cartilages (ls, Fig. 40 View FIG ). There is, however, some resemblance between the “head stains” of E. longaevus and the distribution of the mucocartilage in the snout of larval lampreys, which is distributed into a large median plate and smaller, paired lateral plates ( Damas 1935, 1944; Johnels 1944; Mallatt 1996).
The question of whether the anal fin radials are paired or unpaired remains unanswered, until more significant material turns up, but we have chosen here the most parsimonious interpretation; that is, there is a single, median series of radials, but part of them have been shifted forwards in MHNM 01-123.
The fin radials that extend along the ventral margin of the body, underneath the branchial apparatus, seem to be paired, on account of MHNM 01-125 (pfrad1, pfrad2, Fig. 32 View FIG ). This distribution of the paired fin radials strikingly recalls the position of the paired fins of the anaspid Pharyngolepis oblongus , as reconstructed by Ritchie (1964: fig. 1B), and assumed to be also present in Jamoytius kerwoodi ( Ritchie 1960, 1968; Freedman 1996, 1998). Such ribbon-shaped, elongated ventrolateral paired fins have been regarded by Arsenault & Janvier (1991) and Janvier (1996a, c) as a general character of a clade including anaspids, lampreys, Euphanerops , Endeiolepis and Jamoytius . These paired fins were thus assumed to have been lost in lampreys and much reduced or lost in most anaspids. E. longaevus raises the question of the homology of these paired fins. Clearly, they possessed radials and were thus not a mere skin fold, but they strangely extended ventral to the branchial apparatus ( Fig. 40 View FIG ). This is a unique condition among vertebrates, with the possible exception of certain acanthodians ( Hanke & Wilson 2006). The paired fin endoskeleton and musculature of living gnathostomes is derived from the lateral plate mesoderm and its development is constrained posteriorly to the branchial apparatus and anteriorly to the anus ( Coates & Cohn 1998; Coates 2003). The extension of paired fins all along the body, behind the branchial appartus, in Pharyngolepis was thus already a problem per se, but the presence of such paired fins ventral to the branchial apparatus in E. longaevus further complicates this problem and somehow defies the current rules of fin development. We suggest here that the paired fins of anaspids may be the homologue of the pelvic fins of the jawed vertebrates, as once suggested by Mark Wilson (unpublished communication at meeting, London 1999; see also Hanke & Wilson 2006; Wilson et al. in press), and extended forward along a narrow, median ventral anterior prolongation of the postbranchial trunk musculature, comparable to the hypobranchial musculature of lampreys. This does not necessarily imply serial homolgy of pectoral and pelvic fins, as once suggested by Tabin & Laufer (1993), but only that pelvic fins may be a more general character than previously believed, and were lost in a number of benthic stem gnathostomes.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Genus |
Euphanerops longaevus
| Janvier, Philippe & Arsenault, Marius 2007 |
Achanarella
| Newman 2002 |
Jamoytius
| White 1946 |
E. longaevus
| Woodward 1900 |
E. longaevus
| Woodward 1900 |
E. longaevus
| Woodward 1900 |
E. longaevus
| Woodward 1900 |
E. longaevus
| Woodward 1900 |
E. longaevus
| Woodward 1900 |
E. longaevus
| Woodward 1900 |
E. longaevus
| Woodward 1900 |