Paralimnadia stanleyana ( King, 1855 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4161.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:8B9BDEA7-5F2B-465C-B2A8-757B733CCCE7 |
|
DOI |
https://doi.org/10.5281/zenodo.4685579 |
|
persistent identifier |
https://treatment.plazi.org/id/03E4878E-FFC5-FFF1-FF70-07711635F84A |
|
treatment provided by |
Plazi |
|
scientific name |
Paralimnadia stanleyana ( King, 1855 ) |
| status |
|
Paralimnadia stanleyana ( King, 1855) View in CoL
( Figs. 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 )
Limnadia stanleyana King, 1855: 70 View in CoL ; King, 1864, pl.11, 3 unnumbered figs.; Claus, 1872: 355 –364, pl. 29–30; Brady, 1886a: 83 (list); Whitelegge, 1889: 318 (list); Daday, 1925: 150 (key), 163–166, fig.118; Bishop 1967c : figs. D, 2, 3, 4b; Bishop, 1968a : figs. 4, 7; Webb & Bell, 1979; Brtek, 1997: 58 (list); Richter & Timms, 2005: 348 View Cited Treatment (text).
Eulimadia (sic) stanleyana View in CoL .— Sars 1895: 16 –28, pl.2–3.
Paralimnadia stanleyana View in CoL .— Sars, 1896b: 15; Sayce, 1903: 248 –249, pl.34, fig. 2a–b; Wolf, 1911: 254 (list), 270 (text); Dakin, 1914: 295 (list); Henry, 1924: 121 (list), 133 (text); Rogers et al., 2012: 838.
Type material. Neotype: AM P98989 (ex AM G5223), Maroubra Bay , New South Wales, Sydney, 33°57’S, 151°15’E, no data on date or collector GoogleMaps
Other material examined. New South Wales: Sydney , Botany Bay , 33°58.19’S, 151°11.2’E, G.E. Hine, 5- 10-1920, 1 specimen, AM P4894; Sydney, Maroubra, small pools, puddles and drains, 33°57’S, 151°15’E, 10-6- 1917, F.L. Grutzmacher, 10 specimens, AM P4145; Sydney, Maroubra Bay, 33°57’S, 151°15’E, no data on date or collector, 15 specimens, AM G5223; Sydney, Maroubra, freshwater pans, 33°57’S, 151°15’E GoogleMaps ; 13-4-1924, G.P. Whitley, 2 specimens, AM P53629; Kanangra Boyd National Park , Boyd Hill Swamp, 33°57.05’S, 150°01.49’E, 18-11-1990, E. Albertson, K. Attwood, B. Oldmeadow & G. Wilson, 62 specimens, AM P55707 GoogleMaps ; Kanangra Walls , rockpool, 33°59’15”S ; 150°08’54”E, 27 January 1963, J. Bishop , 25 specimens, AM P54052; same place, 20 December 1963, no collector recorded, 7 specimens, AM P55643; same place, 15 March 2010, BVT, 25 specimens, AM P98990; Pearl Beach , Warrah Sanctuary, 33°33’S, 151°18’E, 16 June 1963, no collector listed, 12 specimens, AM P55641 GoogleMaps ; Pearl Beach , Warrah Biological Station, 28 February, 1963; no collector listed, 22 specimens, AM P55644 ; Bundeena , ocean cliffs 1.7 km southeast, rockpool, 34°05’53”S, 151°09’38”E, 22 March 2014, BVT, 42 specimens, AM P98991 GoogleMaps ; Jerrawangala Lookout near Nowra-Braidwood Rd, rockpool, 35°06’11”S, 150°24’27”E, 6 April 2014, BVT, 30 specimens, AM P98992 GoogleMaps ; Mt Hay , 33°37’18”S, 150°24’29”E, 16 March 2014, J. Porter, 19 specimens, AM P98993 GoogleMaps ; Mt Sturgess , 18 March 2006, J. Meyer, 4 specimens, AM P98994 .
Diagnosis. Egg ( Fig. 5 View FIGURE 5 A–E) subspherical with 2–4 bands of parallel rectangular vertical depressions centred on grooves, depressions with minor protuberances where walls meet join. Male telson with about 8–13 in posterior spine rows (usually about 11) and cercopod with 4–9 (usually 6–8) short spines.
Description. Male. Head ( Fig. 1 View FIGURE 1 B) with ocular tubercle prominent and rounded with compound eye occupying about 60% of its diameter. Asymmetrical dorsal organ immediately to its posterior about two-thirds tubercle height and separated from it by about height of dorsal organ. Rostrum at about 90° to tubercle, broadly rectangular with asymmetrical apex and protruding slightly more than tubercle. Oval ocellus, smaller than compound eye, located dorsally at base of rostrum.
First antenna ( Fig. 1 View FIGURE 1 B) a little longer than peduncle of second antenna and with about 6 lobes, each with many minute sensory setae. Second antenna with dorsal flagellum with 10 antennomeres and ventral flagellum with 11 antennomeres. Most antennomeres with about 3 dorsal spines largely terminal, and about 5–6 setae ventrally ( Fig. 1 View FIGURE 1 D). Basal and apical antennomeres with most divergent numbers of spines and setae, range 1–3 spines and 3–7 setae.
Carapace ( Fig. 1 View FIGURE 1 A) oval, brown dorsally and pellucid ventrally. Dorsal margin unevenly convex with highest part about one quarter from anterior. Abductor muscle at about 45° to carapace long axis. Many growth lines, hardly visible in places. Trunk dorsum with last 10 segments with short spine medially.
Thoracopods. Eighteen pairs of thoracopods. Claspers ( Fig. 1 View FIGURE 1 E) with palm with prominent triangular projection distomedially. Apical club spherical with many stout denticles in dorsomedial gripping area and few long spines medially. Small palp with many short thin spines apically. Finger arcuate with blunt apex and many rounded pits ventrally. Clasper I bearing long palp of 2 palpomeres on medioapical edge of palm, and clasper II in similar position, bearing palp of 3 palpomeres. Palpomere junctions lack spines, and both palps distally with numerous marginal setae on flattened palaform apical area. Long palp of first clasper about 1.25× length of palm and palp of second clasper about 1.5× palm length.
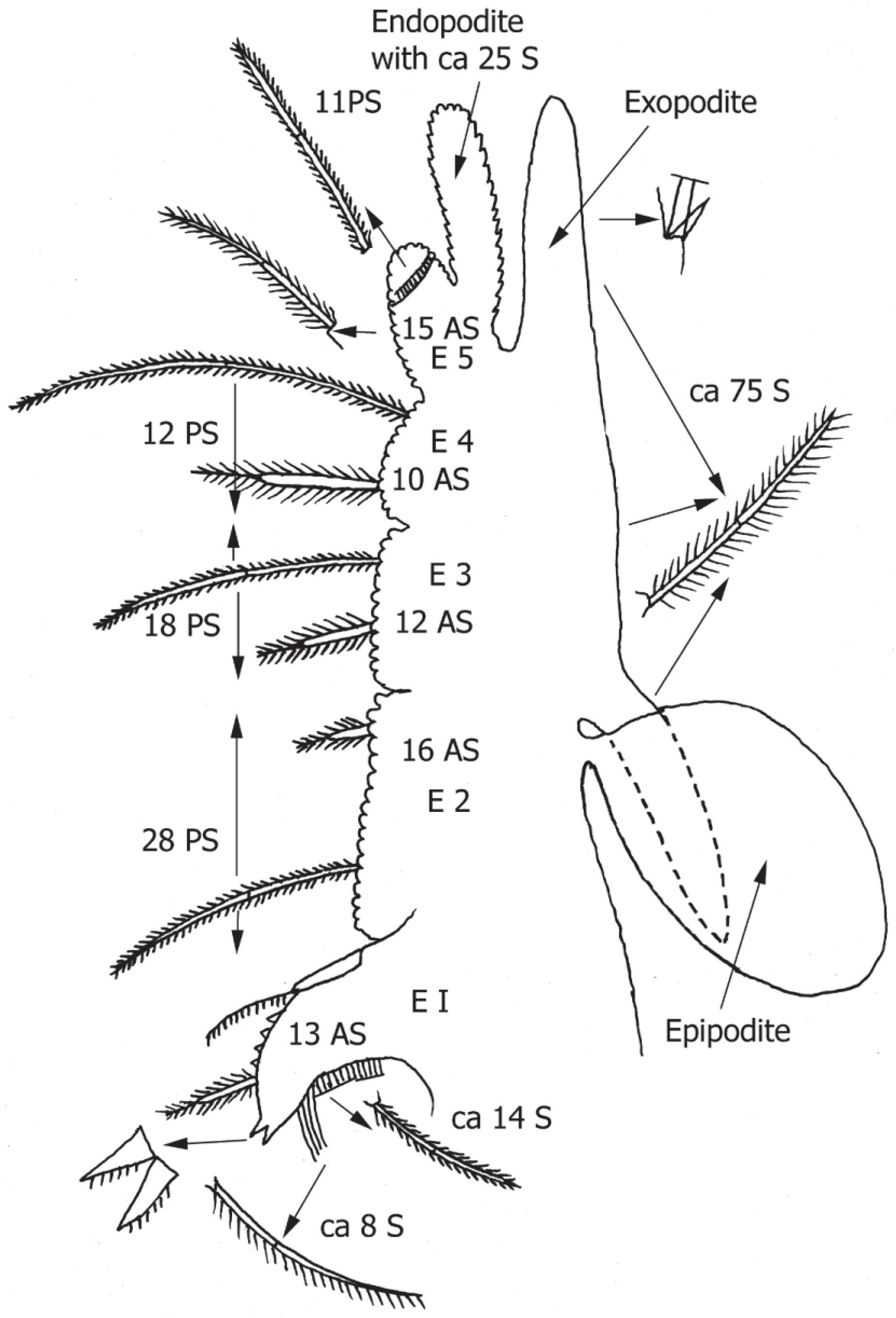
Fifth thoracopod ( Fig. 2 View FIGURE 2 ) with 5 endites medially, the first gnathobase with about 22 setae basally and set of about 13 anterior setae medially, all setae geniculate. Basal setae in 2 groups of 2 different types: about 14 plumose setae with short setules and distally about 8 setae with one-sided pecten of longer setules. Apex of gnathobase with 2 thick, short spines each with few setules on the ventral surface. Upper medial surface of gnathobase with about 4 more thick short spines, about 13 anterior setae and another seta distally with one-sided pecten. Endite 2 with about 16 anterior setae and 28 posterior setae. Endite 3 with about 12 anterior setae and about 18 posterior setae Endite 4 with about 10 anterior setae and about 12 posterior setae. All setae geniculate, posterior setae with shorter setules than anterior setae and proportional lengths of 2 types of setae vary on each endite: anterior setae one-quarter length of posterior setae on endite 2, half length on endite 3, and two-thirds length on endite 4. Endite 5 with about 15 anterior setae, and 11 posterior setae, latter in oblique ring around distal third of endite. Endopodite with about 25 setae and functionally dorsal branch of exopodite subequal in length and bearing many setae. Functionally ventral branch of exopodite also bearing many setae and shorter than oval epipodite.
Third and fourth thoracopod similar but endopodite palp with many short setae distally. Other thoracopods similar to fifth, but shorter and simpler after tenth.
Telson ( Fig. 1 View FIGURE 1 C) with about 11 pairs of spines in the posterior rows, with anteriormost spine a little larger than the next few, remainder even in size and placement. Spines with few spinules.Telsonic filaments originating from mound little higher than the dorsal floor of telson, positioned near third spine. Dorsal floor of telson posterior to mound with steep declivity to slightly convex surface leading to base of cercopod. Cercopods slightly shorter than dorsum of telson, basal 40% with about 8 spines shorter than basal diameter of cercopod, and apical 60% narrowing evenly to sharp apex and bearing many small denticles. Transition marked by small spine. Some spines slightly longer and plumose, others spines shorter and naked. Ventroposterior corner of telson rounded, and hardly protruding.
Females from the Maroubra site. Head ( Fig. 1 View FIGURE 1 G) similar to that of males, but with smaller rounded rostrum and only about 3–5 lobes on first antenna. Carapace ( Fig. 1 View FIGURE 1 B) in mature individuals more vaulted dorsally, often brown all over or at least in upper part; usually with many growth lines, often about 7–9. Trunk with 1 7 or sometimes 18 segments, with usual spines/setae on dorsum of segments X–VII, thoracopods of segments IX and X with dorsal flagellum to hold eggs. Telson ( Fig. 1 View FIGURE 1 H) with about 15 spines (range 14–18) of various sizes; telsonic setae usually inserted after 3rd spine; cercopod with about 8 spines of various lengths, all shorter than basal diameter of cercopod; longer spines fully plumose, shorter ones plumose only apically.
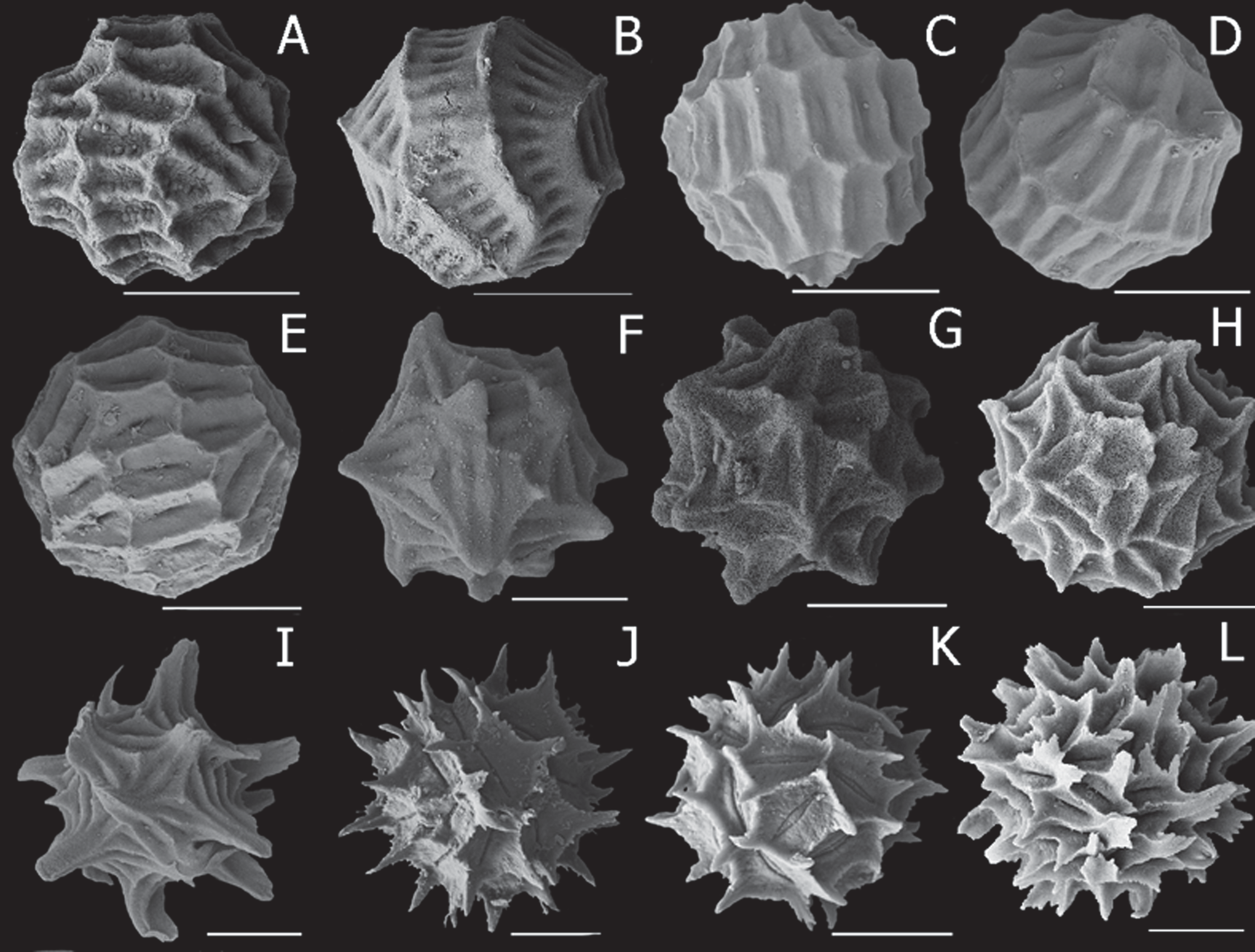
Egg ( Figs. 5 View FIGURE 5 A–E). Mean diameter 212 µm (range 204–213 µm, n =5). Spherical, encircled by two or three bands, plus one to two incomplete bands, of parallel rectangular depressions, each centered on elongated groove about 25–40 µm long. Margins of rectangles generally rounded; minor protuberances in places where ends meet with adjacent band. Sars (1896: pl. 2, figs. 10–12) shows these features coarsely.
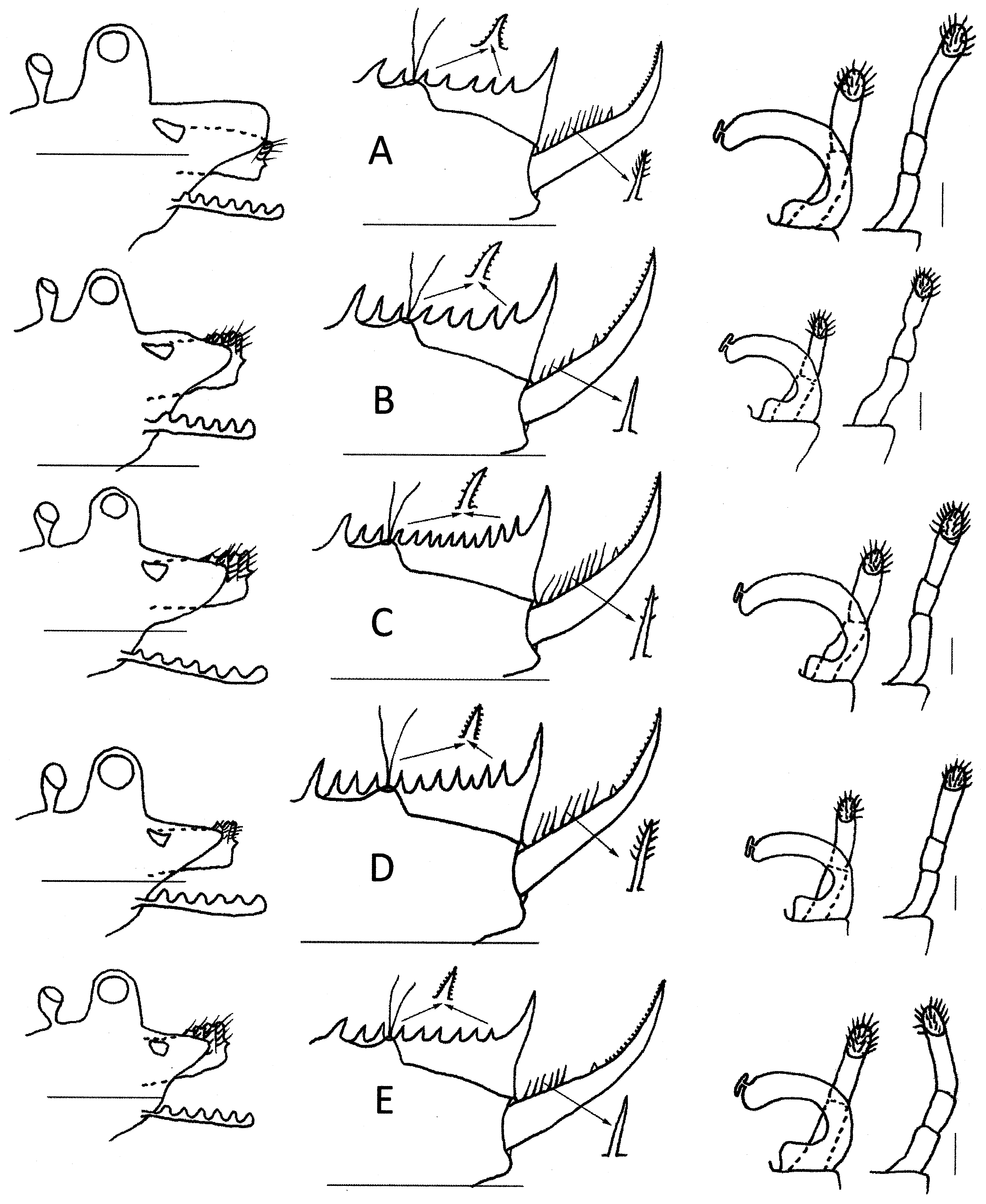
Variability. This is considered by examining between 5 and 8 males from each of five sites (Bundeena, AM P98991; Jerramangala Lookout, AM P98992; Kanangra Walls, AM P98990; near Mt Hay, AM P98993; Warrah Biological Station, AM P55644) in the distribution area and then drawing a typical individual ( Figs. 3 View FIGURE 3 , 4 View FIGURE 4 insert, Tab. 1 View TABLE 1 ). Generally, the rostrum protrudes a little more than the ocular tubercle and while it tends to be triangular, its thickness varies; at Bundeena it is roughly rectangular. The first antenna is always slightly longer than the peduncle of the second antenna and the lobule numbers vary between 6 and 7. The number of antennomeres of undamaged second antenna also varies in the dorsal flagellum (10 or 11) and in the ventral flagellum (11 or 12). The number of telsonic spines varies greatly, from 7 to 13, and also the placement of the telsonic setae from between second and third spine to between fourth and fifth spines. The spines may have few to many denticles but are inerm. The cercopod always has entire spines rather than articulated plumose setae as in most Paralimnadia , but numbers vary from 4 to 9, as does their adornment from being inerm to having many setulae. However, the spines are either subequal to or shorter than the basal cercopod diameter. Finally the claspers show little variation; all have a prominent triangular projection dorsomedially on the palm and palps not much longer than the palms, and the second palp is almost always 3-articulate, but in one case, this palp was only 2-articulate. The carapace varies from being completely opaque and brown to being pellucid, except for a line of brown dorsally.
Antennomeres, on dorsal and 10 and 11 10 and 12 11 and 12 10 and 11 10 and 11
ventral flagella
Telsonic spines 7 8 13 11 9 Bishop (1967a) studied the variability in the number of telsonic spines within and between pools and between four widely separated sites (Kanangra, Warrah, also North Head and Belrose). He noted a range of 6 to 17 in 494 specimens which was not environmentally determined, but which he thought was more likely genetically explained.
Given the above analysis, King’s (1855) characterisation of P. stanleyana as having a large palp with two palpomeres and the telson with about 11 posterior row spines is clearly too narrow a description. Sars’ (1896) more detailed description mentions 10 ‘dorsal spines’ in the posterior spine row and confirms the triple articulation of the large palp of the second clasper. His drawings ( Fig. 1 View FIGURE 1 J–L) show a palp of three palpomeres, a first antenna with five lobes, and an equilateral triangular rostrum. This variability P. stanleyana is confounding.
Remarks. This species was the first limnadiid clam shrimp described from Australia and is often mentioned in early papers. The brief description by King (1855) was enlarged by Claus (1872) and well expanded and illustrated by Sars (1895) (who misspelt Eulimnadia as Eulimadia). Although Sars (1895) concept of P. stanleyana is used today, the brevity of King's original description could apply to other species of the genus including P. s o rd i d a ( King, 1855), which occurs in the same area and itself lacks type material. The original material of P. stanleyana , which by default constituted the type material, is lost. The original collecting site is at Coogee, 3 km north of previous environs similar to Maroubra ( King 1855), but no collections exist from this site and the area has long been completely modified to remove any swamps or temporary pools. Therefore, a neotype corresponding to Sars (1895) concept of P. stanleyana is herein designated from Maroubra Bay to fix the identity of the species in line with common usage.
Given the brief and partly erroneous characterisation of P. sord i d a and P. stanleyana by King (1855), and the occurrence of both species in the same area, the question of their validity needs to be addressed. Both have claspers with large palps of three palpomeres and there is too much variation in the numbers of telsonic spines to provide reliable differentiation. However, the two species are easily distinguished by (a) eggs with bands of rectangular depressions in P. stanleyana as opposed to eggs of 16–20 small rounded knobs, each subtended by a few (ca. 6–8) groove-ridge combinations in P. s o rd i d a, (b) cercopod with 4–9 spines of length shorter than cercopod basal diameter in P. stanleyana compared with about 12 two-articled plumose setae about 1.5 × longer than cercopod basal diameter in P. sordida , (c) male first antenna with about 6 or 7 lobes in P. stanleyana compared with about 9 lobes in P. s o rd i d a, (d) no spines at palpomere junctions in P. stanleyana as opposed to 3–5 spines (but only at the basal junction in the large palp) in P. s o rd i d a.
Sars (1895) suggests P. stanleyana might be synonymous with Estheria compressa Baird, 1860 , from India. This is untenable, however, as E. compressa has a spine at the ventroposterior corner of the telson ( Rogers & Padhye 2015; Rogers et al. 2016) and its egg is very different, with the surface consisting of pentagons rather than bands of rectangular depressions ( Rabe 2010; Rogers et al. 2016).
Distribution and ecology. Paralimnadia stanleyana occurs throughout the Sydney basin, currently only in pools (= gnammas) on sandstone surfaces ( Timms 2006) ( Fig. 5 View FIGURE 5 ). At European settlement it also occurred in sandy substrates of the Botany swamps and among coastal dunes at Coogee and Maroubra ( King 1855; Sayce 2003; Henry 1924), but these sites have since been fully urbanised, drained (beach dunes, some Botany swamps) or made permanent (some swamps at Botany).
Much is known about the ecology of this species due to the work of Bishop (1967a, b; 1968a, b; 1969). In summary, P. stanleyana inhabits temporary rainwater pools that fill unpredictably and irregularly. Its eggs diapause during the dry winter and remain quiescent until temperatures rise and water is present. Active stages are only present during warmer months. In the Kanangra pools, nauplii eat Gymnodinium and immatures eat Scenedesmus . Larvae grow quickly and adults can live up to four months, but usually die sooner because pools dry. Populations cannot survive in pools that do not contain water long enough for the species to accumulate a reserve of eggs. Only some eggs hatch with each pool filling because, according to Bishop (1967b) , only some are in a position where darkness and low oxygen are not inhibitory. Dispersal powers are low ( Bishop 1967b ).
TABLE 1. Variation in some morphological characters in males of P. stanleyana from five sites around Sydney (see also Fig. 2).
| Character | Bundeena | Jerrawangala | Kanangra | Mt Hay Warrah |
|---|---|---|---|---|
| Rostrum | almost rectangular | broadly triangular | broadly triangular | narrow triangular medium triangular |
| Lobes on first anntenna | 7 | 6 | 7 | 6 6 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Paralimnadia stanleyana ( King, 1855 )
| Timms, Brian V. 2016 |
Paralimnadia stanleyana
| Rogers 2012: 838 |
| Henry 1924: 121 |
| Dakin 1914: 295 |
| Wolf 1911: 254 |
| Sayce 1903: 248 |
| Sars 1896: 15 |
(sic) stanleyana
| Sars 1895: 16 |
Limnadia stanleyana
| Richter 2005: 348 |
| Brtek 1997: 58 |
| Daday 1925: 150 |
| Whitelegge 1889: 318 |
| Brady 1886: 83 |
| Claus 1872: 355 |
| King 1855: 70 |