Parartemia boomeranga, Timms, Brian V, 2010
|
publication ID |
https://doi.org/ 10.5281/zenodo.199709 |
|
DOI |
https://doi.org/10.5281/zenodo.6200938 |
|
persistent identifier |
https://treatment.plazi.org/id/5D556922-6448-6555-FF0B-A657FBCEFBC9 |
|
treatment provided by |
Plazi |
|
scientific name |
Parartemia boomeranga |
| status |
sp. nov. |
Parartemia boomeranga View in CoL sp.nov.
(Figs. 4,6,7)
Parartemia n sp c Timms & Savage, 2004, p 22, 26, 35.
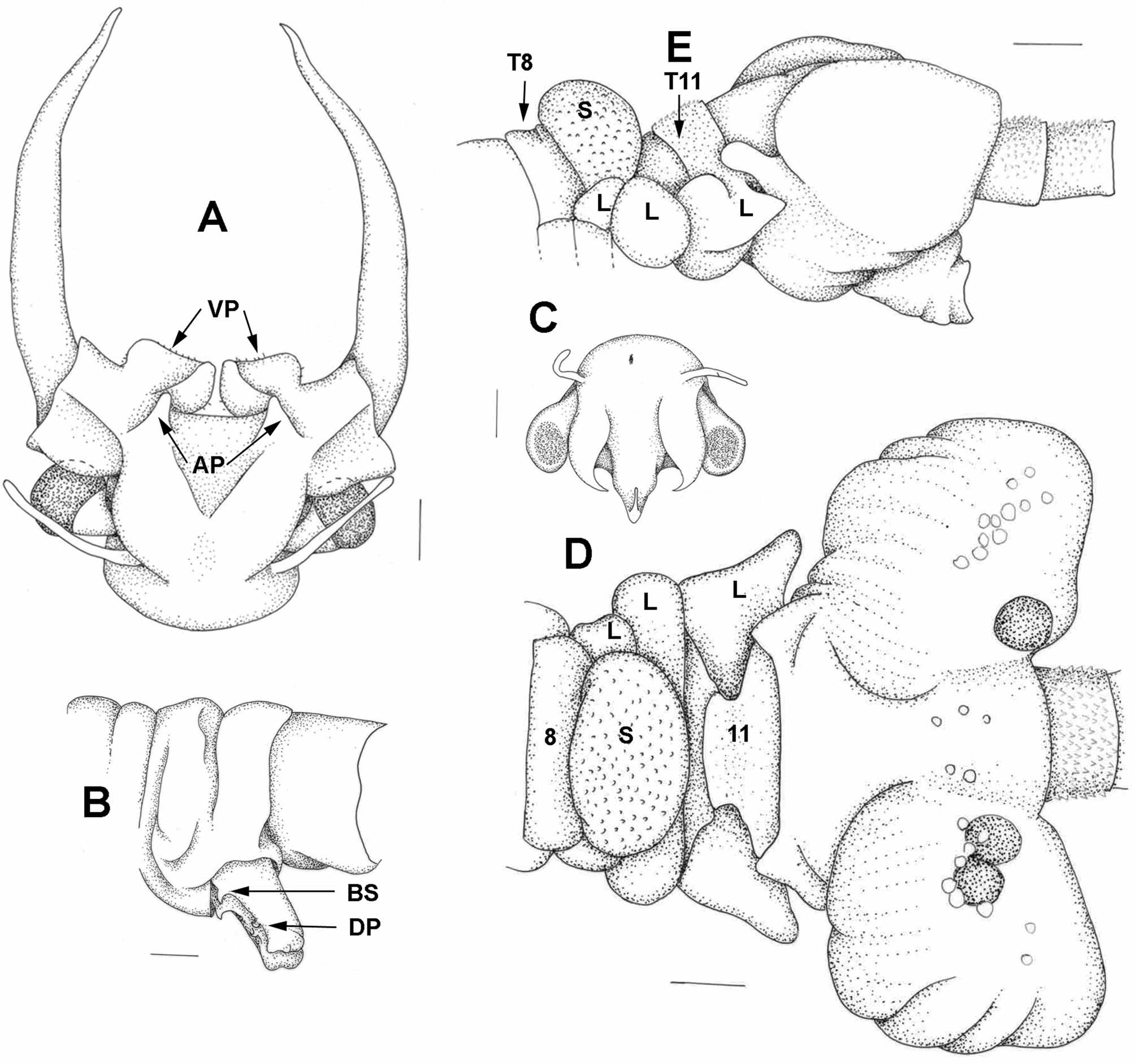
Etymology. The specific name is suggested by the boomerang-shaped thick area (short arms at an angle and thickened mid region) on the medial surface of the ventral processes. These fit snugly against the female 11th thoracopod semispherical bases in amplexus.
Holotype. One male, unnamed lake on Gunyidi - Wubin Road, 15.5 km E of Gunyidi, (30o 07’ 08”S, 116o 14’ 36”E), 14 September 2003, BVT, WAM 45211.
Allotype. One female, same collecting data as holotype, WAM 45212.
Paratypes. Two males, two females, some collecting data as holotype, WAM 45213; two males, two females, same collecting data as holotype, AM P82972.
Description. Male. Length 22.2 mm (head plus thorax 9.1 mm, abdomen 13.1 mm).
First antenna ( Fig 6 View FIGURE 6 A) filiform, about 1.5 times length of eye plus peduncle.
Second antenna. Basal antennomeres ( Fig 6 View FIGURE 6 A) fused at an angle of about 45o from body axis. Ventral margin with paired ventral processes ( Fig 6 View FIGURE 6 A) about 2.5 times longer than deep. Distoventral corner of ventral processes broadly rounded and slightly protruding, ventral margin weakly concave and medial margin curved asymmetrically with maximum protrusion about two thirds distance from ventral margin and inner margin well indented into the ventral process, so that whole medial portion of the ventral process unsupported from the ventral margin of the basal antennomere. However this portion with a boomerang-shaped thickened area. Lateral and ventral margins of ventral process clothed with well spaced small spines. Medial area between ventral processes concave. Anterior processes ( Fig 6 View FIGURE 6 A) digitiform, small and length about equal to depth of ventral processes. Distal antennomere ( Fig 6 View FIGURE 6 A) about twice length of basal antennomere, generally curved medially and tapering to a sharp apex, but apical portion convexly curved and with a tumidity mesially.
Thoracic segments with small lateral lobes, increasing in size posteriorly and reaching a maximum on segment 11. Lateral bulge on first genital segment even greater than 11th lobe, so that maximum body width in genital segments. Eleven pairs of thoracopods, with first two reduced in size and last without an epipodite.
Gonopods ( Fig 6 View FIGURE 6 B) fused basally. Each free apical portion with a basal sharp spine and a ventrolateral short digitiform process at about two-thirds the length of the appendage.
Abdomen with segments increasing in length and decreasing in diameter sequentially 1 to 6, with sixth 1.75 times length and half the diameter of first segment.
Female. Length 15.3 mm (head plus thorax 9 mm, abdomen 6.3 mm).
Head ( Fig 6 View FIGURE 6 C) with first antenna filiform, a little shorter than eye plus peduncle. Second antenna somewhat longer than length of eye plus peduncle, flattened, with parallel sides and a round symmetrical apex bearing a short narrow and pointed appendix. Distinct naupliar eye midway between bases of first antenna. Labrum with a recurved spine.
Thoracic segments ( Figs. 6 View FIGURE 6 D,E) 1–7 normal, segment 8 with a trapezoid dorsum and small lateral lobes, segment 9 with a prominent dorsal swelling and larger lateral lobes, segment 10 reduced with a narrow dorsum and apparently no lateral lobes and segment 11 with a less reduced dorsum and large lobes extended posteriolaterally into a triangular extension and ventrally into a rounded swelling. Dorsal surfaces of segments 10 and 11 sclerotized.
Most thoracopods as in male. First thoracopod (Fig 7A) reduced in size but hardly in relative size of component parts. Both basal anterior basal setae of endites 4 and 5 elongated. Tenth thoracopod (Fig 7B) also reduced in size and in relative size of components. Endites 1+2 and 3 small and with few posterior setae, exopodite semi-oval, basal anterior seta of endite 4 long, but other anterior setae of normal relative lengths. No 11th thoracopod, but distinct base in the form of a semispherical mound.
Anterior edge of first genital segment ( Figs. 6 View FIGURE 6 D,E) a collar with a lateral protrusion reaching anteriorly over lobe of 11th thoracic segment.
Brood pouch ( Figs. 6 View FIGURE 6 D,E) wider than long, so that whole structure approaches three times width: length. Anteriolateral and posteriolateral corners prominent and a shallow groove between them. Lobes joined ventrally into a short tubular structure, directected posteriorly bearing the gonopore.
FIGURE 7. Female thoracopods. A, P. boomeranga sp. nov. 1st thoracopod; B, P. boomeranga sp. nov. 10th thoracopod; C, P. b i c o r n a sp. nov. 1st thoracopod, length; D, P. b i c o r n a n. sp 10th thoracopod; E, P. laticaudata sp. nov. 1st thoracopod; F, P. laticaudata sp. nov. 10th thoracopod; G, P. laticaudata sp. nov. 11th thoracopod; H, P. mouritzi sp. nov. 1st thoracopod; I, P. mouritzi sp. nov. 10th thoracopod; J, P. purpurea sp. nov. 1st thoracopod; K, P. purpurea sp. nov. 10th thoracopod; L. P. purpurea sp. nov. 11th thoracopod; M, P. ve ro n ic a e sp. nov. 1st thoracopod; N, P. veronicae sp. nov., 10th thoracopod. For further explanation on the thoracopods see legend of Fig. 3.
Differential diagnosis. This species is most similar to P. longicaudata , and indeed in an early key ( Timms, 2004), males of the two species were inseparable. However, there are a number of minor differences: (a) the ventral processes are proportionally longer in P. boomeranga sp. nov., (b) the medial edge of the ventral processes is much more convex (boomerang shaped) in P. boomeranga sp. nov. than in P. longicaudata , (c) the medial thickened area in the ventral processes in P. longicaudata is almost straight, while in P. boomeranga sp. nov. it is boomerang shaped, (d) the distal antennomere is evenly concavely curved in P. longicaudata , but in P. boomeranga sp. nov. it is unevenly curved because it is thickened midlength and has outwardly curved apices, (e) the sixth abdominal segment is> twice the length of the first in P. longicaudata , but <twice in P. boomeranga sp. nov., (f) the basal spine of the gonopod is curved in P. longicaudata but straight in P. boomeranga sp. nov. and (g) the distal spine is sited about half way along the gonopod in B. longicaudata but two-thirds the distance in P. boomeranga sp. nov.
While females of the two species ( P. longicaudata , P. boomeranga sp. nov.) share some important characteristics, e.g. the segment 9 large dorsal tumidity and its large lateral lobes, segments 10 and 11 are dorsally sclerotized in both, and there are large ventrolateral tumidities on segment 11 in both. However there are many differences: (a) the first antenna are longer than the eye plus peduncle, and the narrow apical portion of the second antenna is longer than antenna width in P. longicaudata , as opposed to the first antenna being shorter than the eye plus peduncle, and narrow apical portion of second antenna being shorter than its width in P. boomeranga sp. nov., (b) thoracic segment 8 lacks a lateral lobe in P. longicaudata , but has one in P. boomeranga sp. nov., (c) segment 10 is larger than segment 11 in P. longicaudata , but relative size is reversed on P. boomeranga sp. nov., (d) in P. boomeranga sp. nov. segment 11 has large lateral lobes extended posteriorly in triangular projections, and (e) the brood pouch is generally trapezoid in P. longicaudata , but rectangular in P. boomeranga sp. nov.
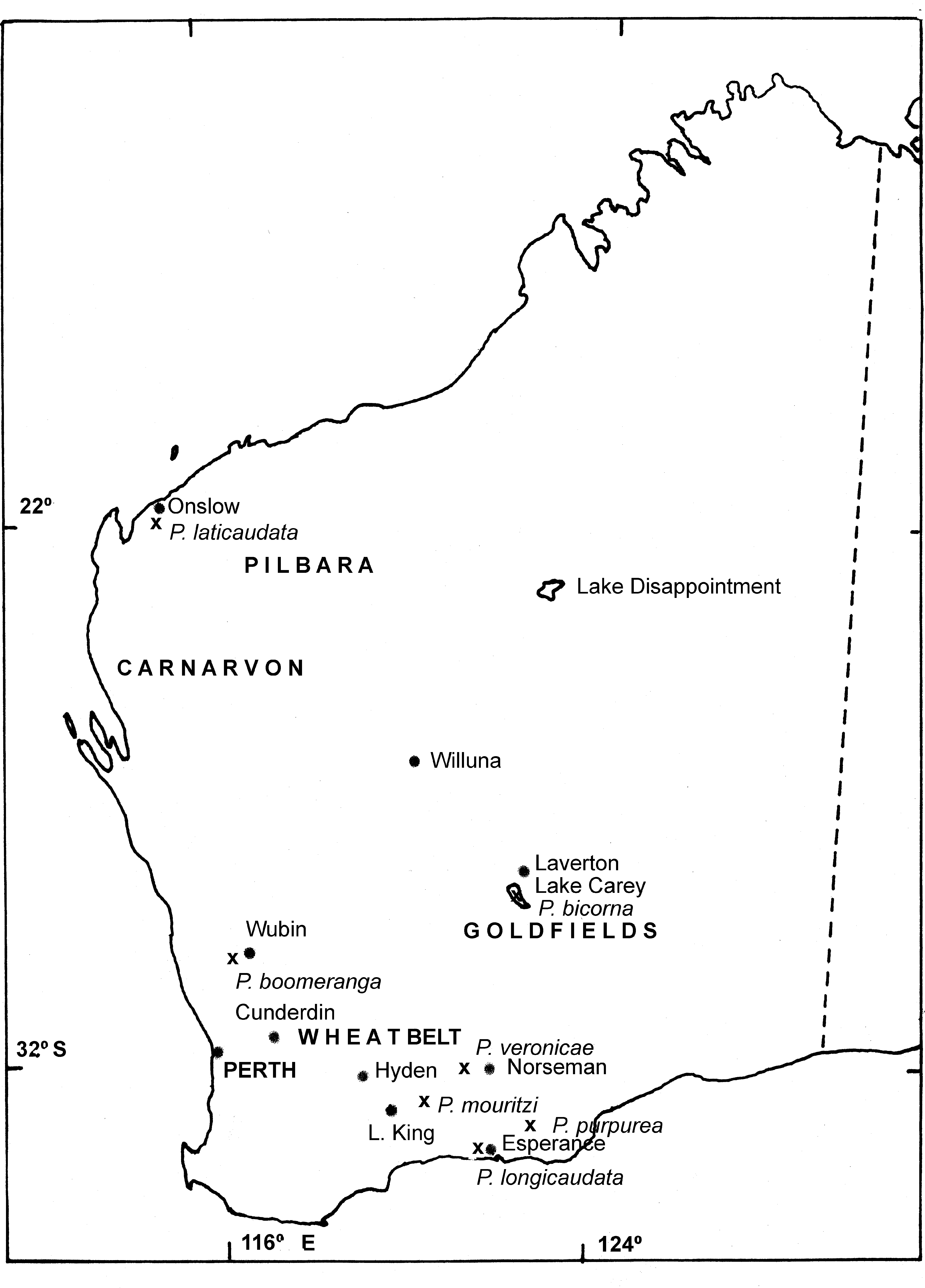
Distribution and ecology. P. boomeranga sp. nov. is known from only a few lakes in a narrow band northeast of Perth from near Gunyidi to Wubin to Kalannie to Cunderin (Timms et al., 2009)( Fig. 4 View FIGURE 4 ). Most of its known sites are salinised and extant populations have not been located 2006–2008, leading Timms et al. 2009) to suggest this species be listed as Vulnerable under ICUN criteria. Little is known of its ecological requirements, other than a maximum salinity record of 120 g /L (Timms et al. 2009).
| WAM |
Western Australian Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.