Solenysa, SIMON, 1894
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2010.00640.x |
|
persistent identifier |
https://treatment.plazi.org/id/038887F0-723A-FFA7-FC6E-FE58FB1BE72D |
|
treatment provided by |
Valdenar |
|
scientific name |
Solenysa |
| status |
|
SOLENYSA SIMON, 1894 View in CoL View at ENA
Solenysa Simon, 1894: 677 View in CoL .
Type species: Solenysa melloteei Simon, 1894 .
Composition: Twelve species, including five new species described here: Solenysa akihisai Tu sp. nov., Solenysa geumoensis Seo, 1996 , Solenysa lanyuensis Tu sp. nov., Solenysa longqiensis Li & Song, 1992 , Solenysa melloteei Simon, 1894 , Solenysa partibilis Tu et al., 2007 , Solenysa protrudens Gao et al., 1993 , Solenysa reflexilis Tu et al., 2007 , Solenysa retractilis Tu sp. nov., Solenysa tianmushana Tu sp. nov., Solenysa wulingensis Li & Song, 1992 , and Solenysa yangmingshana Tu sp. nov.
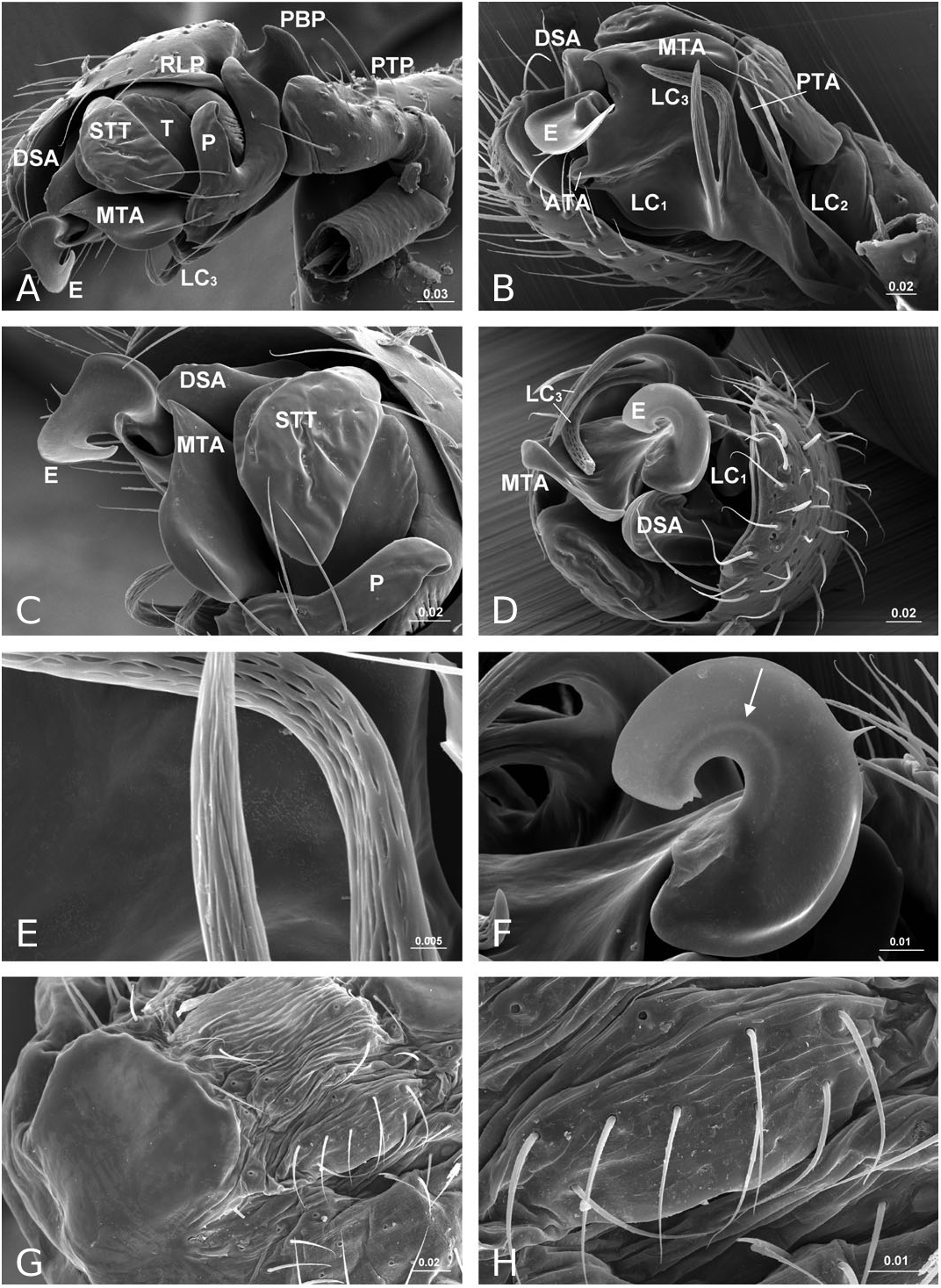
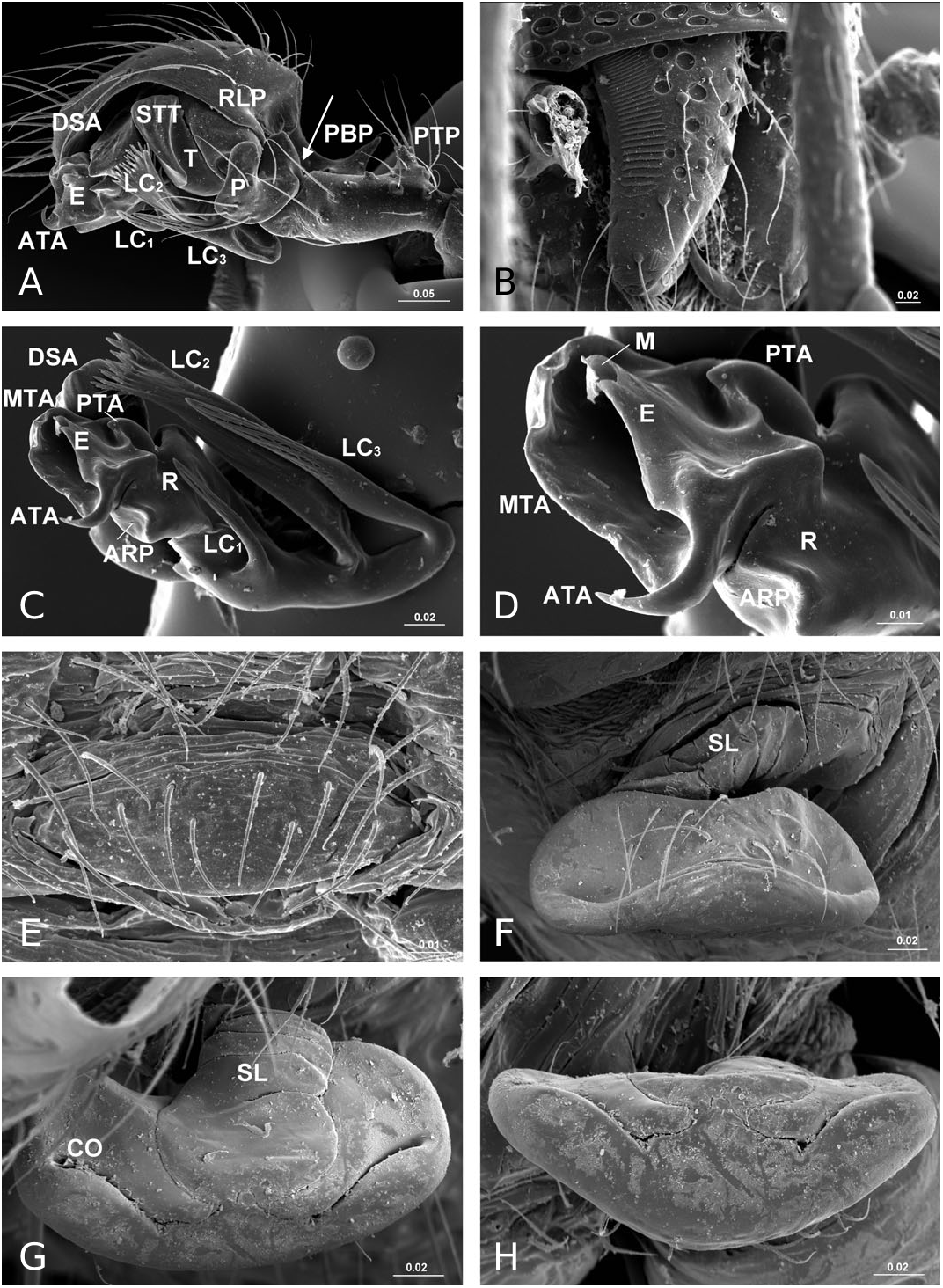
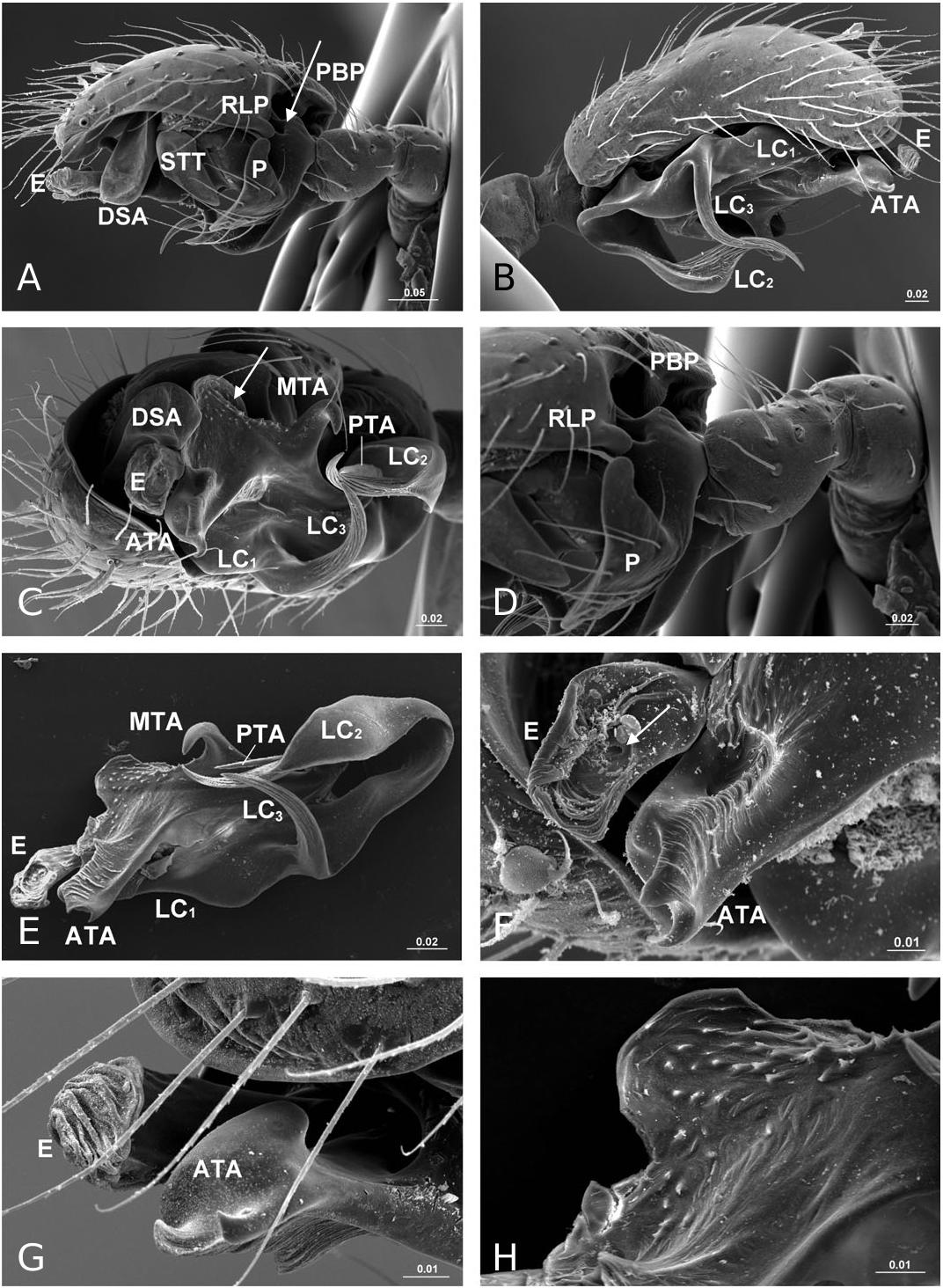
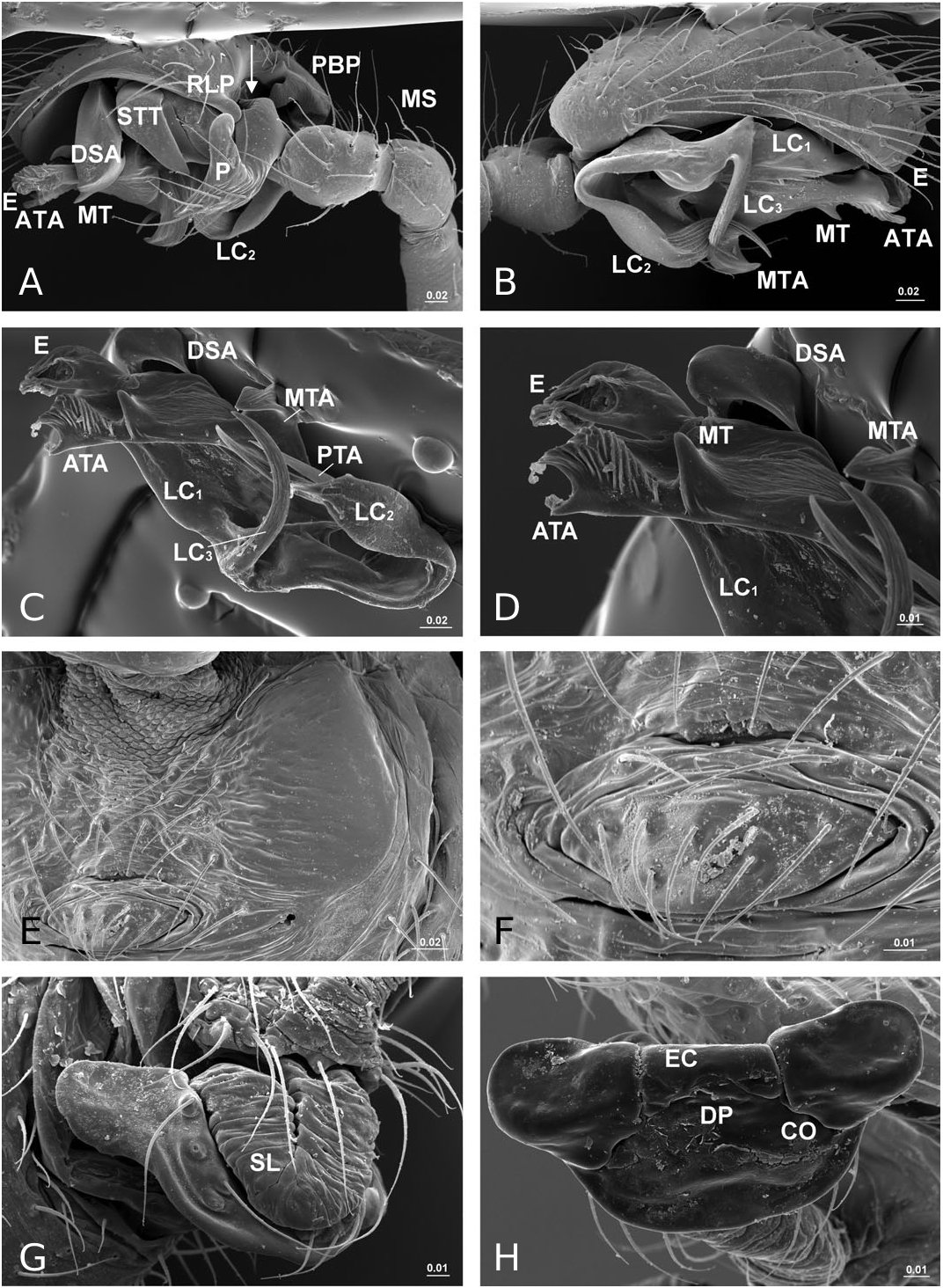
Diagnosis: Solenysa species can be distinguished from all other linyphiids by the four lateral lobes on each side of the carapace and by the tubular-shaped petiole ( Fig. 16A–C View Figure 16 ). Males are also diagnosed by the palpal cymbium, which has a large probasal process and a small retrolateral process that is articulated with the paracymbium ( Fig. 12A View Figure 12 ). Females are also diagnosed by the long membranous solenoid, which connects the epigynum to the abdomen, twists and lifts the epigynum up in nonfunctional stage ( Figs 8H, 13A View Figure 13 ; see also Tu & Li, 2006b: figs 28–30).
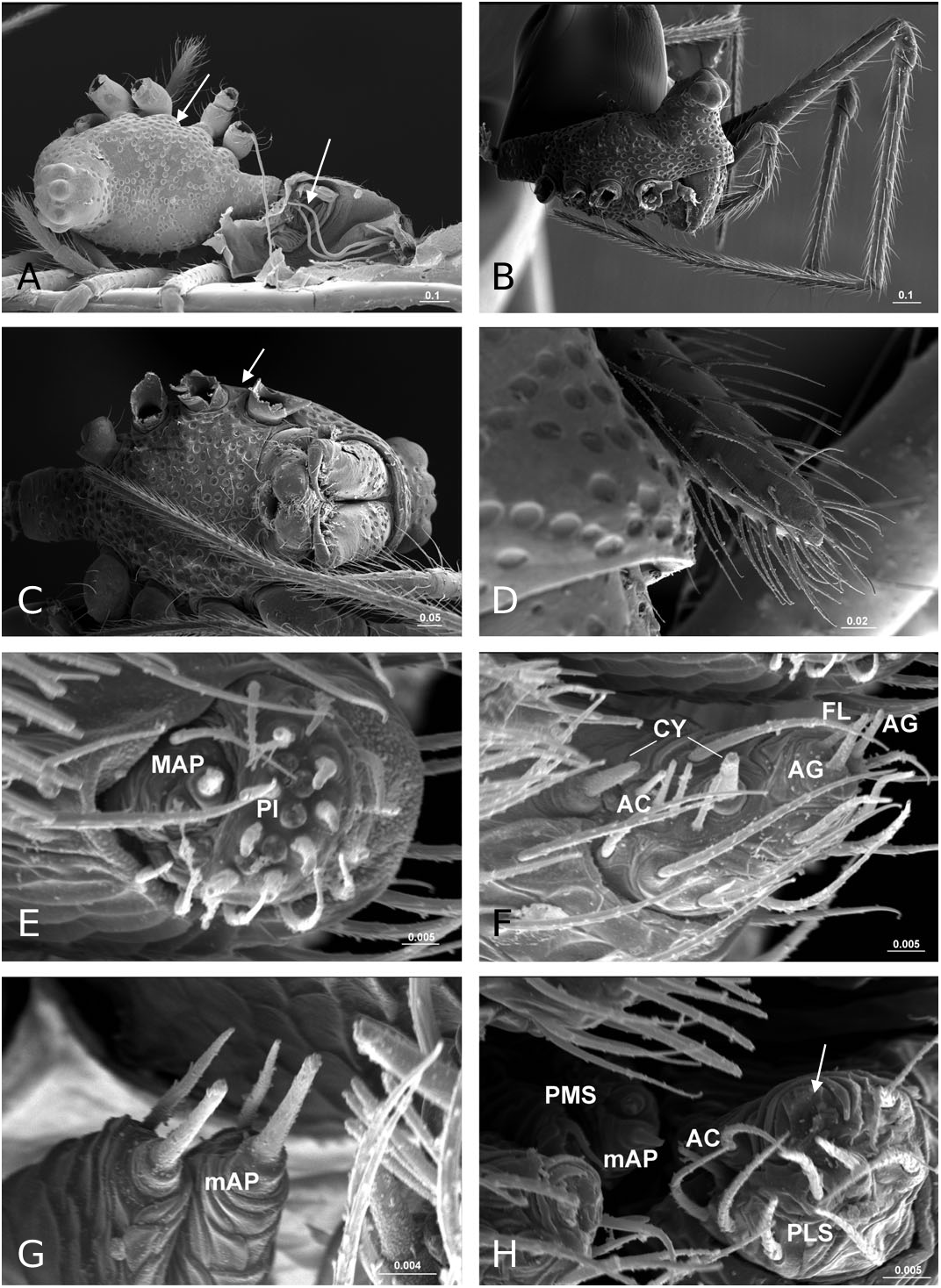
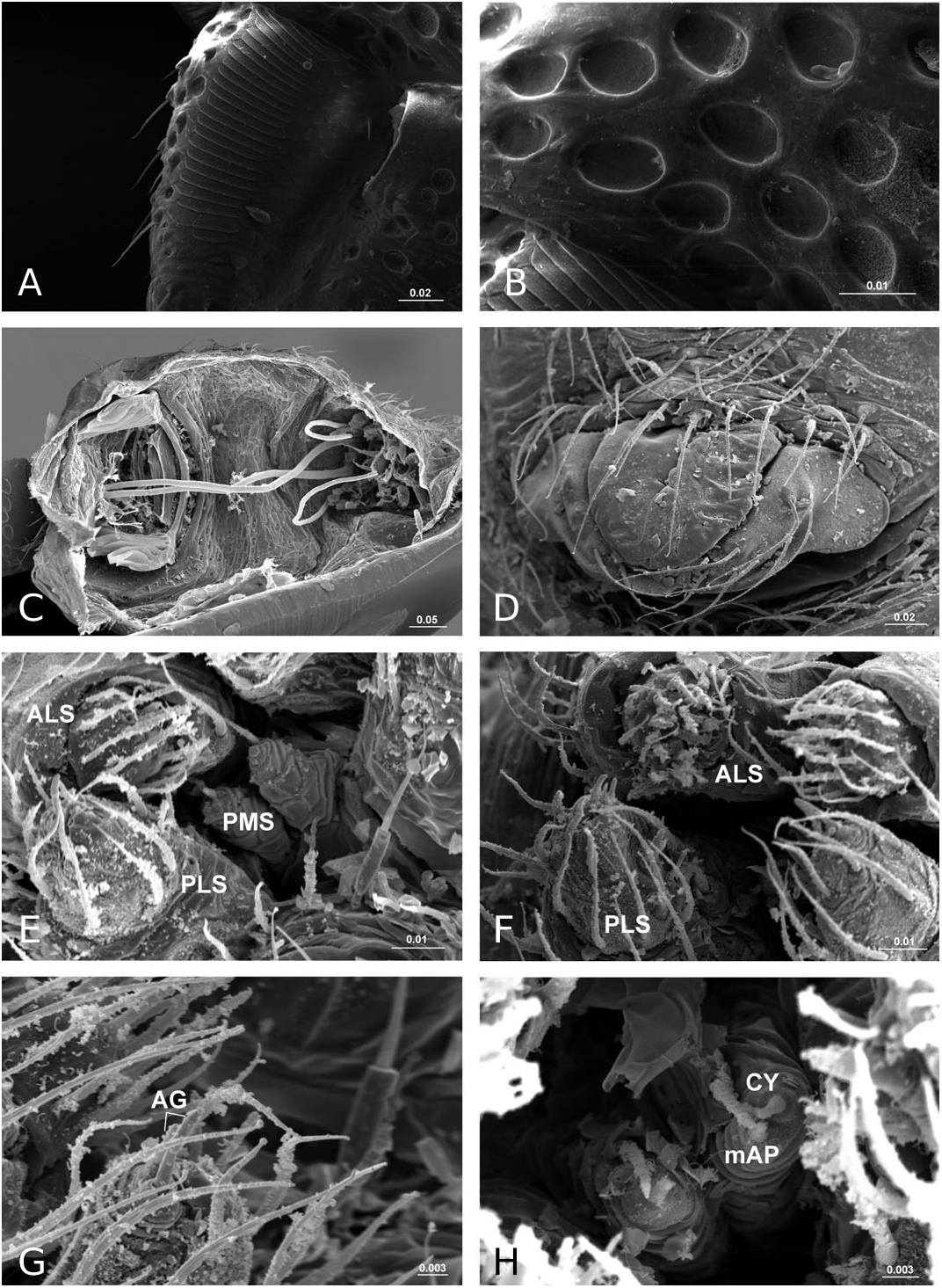
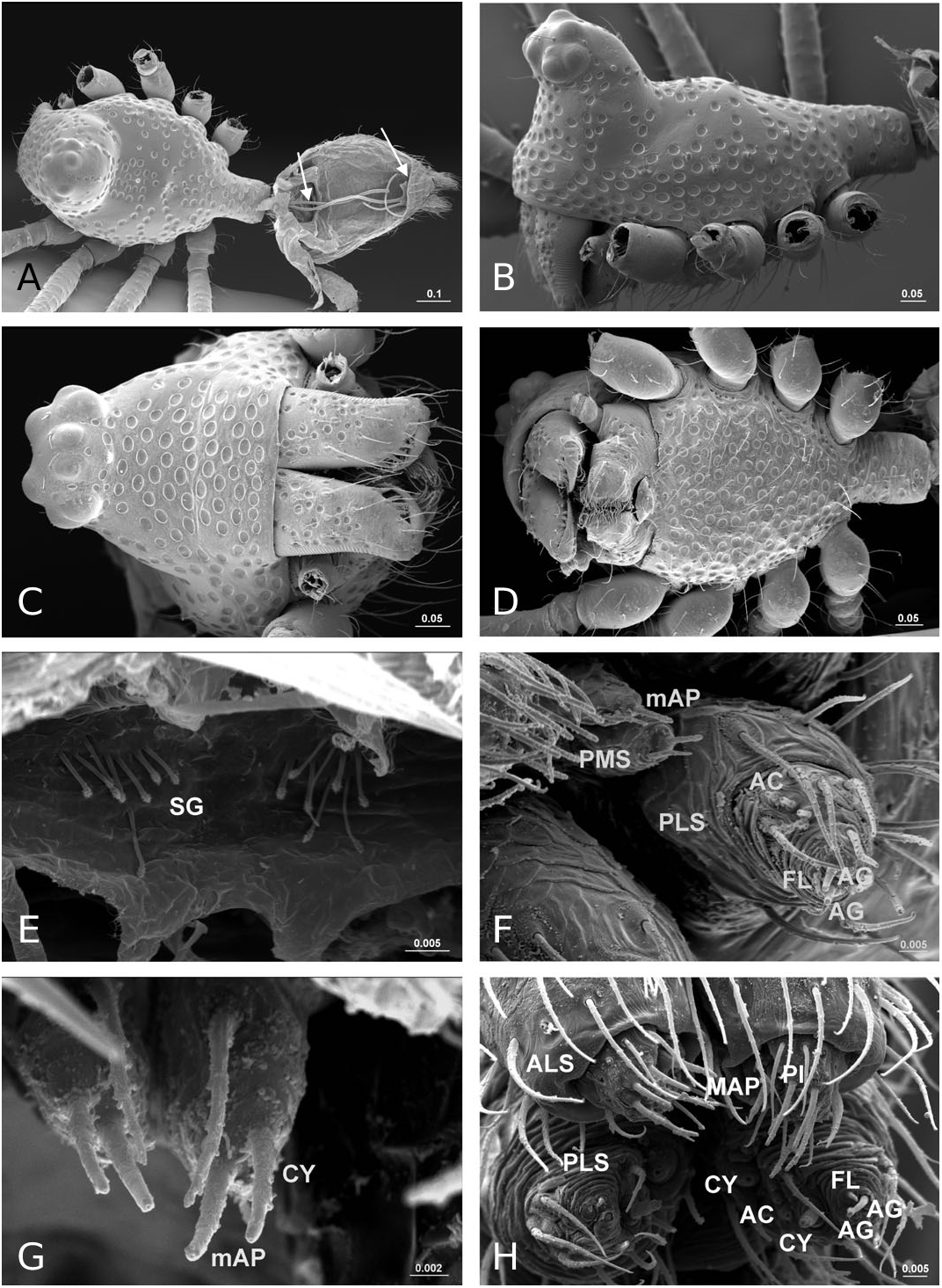
Description: Small spiders, male total length 1.11– 1.61, female 1.27–1.90. Both sexes similar in general appearance with little interspecies variation ( Fig. 16A–C View Figure 16 ): cephalic region of both sexes turret-like, raised bearing AMEs in front, PMEs on top, ALEs and PLEs on its sides; AMEs black and very small, AMEd about half of other eyes, rest of eyes subequal; AER recurved, AME-AME less than AMEd, AME-ALE longer, PER straight, PME-PME equal to PMEd, PME-PLE shorter, ALE and PLE juxtaposed. Many impressed, round pits scattered on carapace and anterolateral surface of chelicerae ( Fig. 18B View Figure 18 ). Posterior part of carapace elongated into a tubular-shaped petiole. Sternum continuous with carapace laterally, leaving four openings at each side through which coxae are articulated. In dorsal view four lobes can be distinguished around the leg coxae ( Fig. 16A–C View Figure 16 ), but not conspicuous in S. longqiensis ( Fig. 14A View Figure 14 ) and S. yangmingshana sp. nov. Legs long and slender, without macrosetae. Patella I–IV with one distal short spine ( Fig. 16B View Figure 16 ). Tm I 0.17–0.26. Tm IV absent. Female palpal claw absent ( Figs 14E View Figure 14 , 16D View Figure 16 ). Book lung cover almost smooth ( Fig. 12G View Figure 12 ). Epiandrous fusules absent in males (studied in S. partibilis , Fig. 12H; S View Figure 12 . longqiensis, Fig. 15H; S View Figure 15 . protrudens, Fig. 17E; S View Figure 17 . retractilis and S. wulingensis , Fig. 20E View Figure 20 ). Tracheal system consisting of unbranched median and lateral trunks; median pair slightly wider in diameter than lateral pair, extending into prosoma through petiole (studied in S. partibilis ; S. akihisai ; S. longqiensis ; S. protrudens , Fig. 16A; S View Figure 16 . retractilis, Fig. 18C; S View Figure 18 . wulingensis, Fig. 20A View Figure 20 ). Spinnerets (studied in S. partibilis ; S. longqiensis ; S. protrudens ; S. retractilis ; and S. wulingensis ): ALS with one major ampullate gland spigot and one nubbin, as well as about 12 piriform gland spigots ( Fig. 16E View Figure 16 ); PMS with one minor ampullate gland spigot ( Fig. 16G View Figure 16 ), cylindrical gland spigot present ( Fig. 20G View Figure 20 ) or not ( Fig. 16G View Figure 16 ); PLS with four aciniform gland spigots between the two cylindrical gland spigots ( Fig. 16F View Figure 16 ); basal part of mesal cylindrical gland spigot slightly larger than distal one ( Fig. 16F View Figure 16 ); flagelliform and aggregate gland spigots (‘araneoid triplet’) well developed in females ( Fig. 16F View Figure 16 ), reduced to nubbins in adult males ( Fig. 16H View Figure 16 ), but present in adult males of S. retractilis ( Fig. 18F View Figure 18 ) and S. wulingensis ( Fig. 20F View Figure 20 ). Male palpal tibia with one prolateral, two retrolateral trichobothria ( Fig. 21A View Figure 21 ). Proximal part of cymbium extending posteriorly and then turning retrolaterally, forming a large excavation and probasal process, protruding out ( Fig. 15A–C View Figure 15 ), or hidden ( Fig. 21A View Figure 21 ). Small retrolateral process anterior to proximal part of paracymbium ( Fig. 12A View Figure 12 ). Paracymbium U-shaped, curved almost horizontally ( Fig. 15B View Figure 15 ) or torsion-shaped turning in the middle ( Fig. 21A View Figure 21 ) with additional curve at proximal part that articulates with cymbial retrolateral process ( Fig. 21A View Figure 21 ). Tegulum with a chitinized triangular structure in retrolateral view, termed here as ‘S olenysa tegular triangle’ ( Fig. 16A View Figure 16 ). Distal suprategular apophysis large, extending ventrally in retrolateral view ( Fig. 19A View Figure 19 ). Radix usually small, combined with other elements of embolic division ( Fig. 15D View Figure 15 ) or even embedded in a large membranous area that connects the basal part of terminal apophysis and lamella characteristica ( Figs 10D, 19E View Figure 19 ). Terminal apophysis with three free ends, normally the median one larger ( Fig. 7). Lamella characteristica deeply divided into three well-developed branches, at least one of them, usually the median branch, ribbon like ( Fig. 7). Embolus short, sclerotized ( Fig. 12F View Figure 12 ), translucent (less sclerotized) or totally membranous ( Fig. 19E, F View Figure 19 ), without pointed embolus proper. No embolic membrane arising from column, but sometimes embolus with membranous projection, the distal embolar membrane (‘M’, see Figs 15F View Figure 15 , 17D View Figure 17 ). Epigynum well sclerotized, encapsulated, hanging at distal end of a long, transversally wrinkled, membranous solenoid ( Fig. 13A View Figure 13 ). Solenoid arising from abdomen tegument, twisting and lifting epigynum up in nonfunctional stage ( Figs 8H, 13A View Figure 13 ; see also Tu & Li, 2006b: figs 28–30). A pair of seams on epigynal dorsal surface; curved ( Fig. 13B View Figure 13 ) or straight ( Fig. 14H View Figure 14 ), transversal ( Fig. 17G View Figure 17 ) or longitudinal ( Fig. 14H View Figure 14 ); distal ends served as copulatory openings, proximal ends as fertilization openings. Fertilization ducts directed mesally ( Fig. 8I).
Remarks: The somatic morphology of Solenysa is quite uniform across species and shows little sexual dimorphism, although some of these features can be found in other linyphiids. For example, various types of cephalic lobes can be found in the males of other erigonine lineages (see Hormiga, 2000: figs 32–35), whereas in Solenysa the cephalic lobe is found in both sexes. The round pits on the carapace and chelicerae of Solenysa are also present in several not closely related taxa [e.g. in Lophomma punctatum (Blackwall) , see Hormiga, 2000: plate 47], but those of Solenysa have a flat bottom, and no seta arises from it ( Fig. 18B View Figure 18 ). The tubular-shaped carapace petiole
·
also can be found in Cresmatoneta mutinensis (Canestrini, 1868) . The simultaneous presence of these and other somatic characters is unique to Solenysa , which makes this genus easily distinguishable from all other linyphiids.
In contrast to other erigonines, the radix in Solenysa is very reduced, to the extent that often it is not easy to distinguish it, and it is either combined with the terminal apophysis ( Fig. 15D View Figure 15 ), or it becomes embedded in the membranous area which connects the basal part of the well-developed terminal apophysis and lamella characteristica ( Fig. 19E View Figure 19 ). In the latter case, observed under the compound microscope, the radix is only a small, chitinized structure in the translucent area between the terminal apophysis and the lamella characteristica ( Fig. 10D). Although this is not discernable in SEM images, it can be dissected out for examination. In fact, in one of our previous studies ( Tu & Li, 2006b), we had totally missed the radix and incorrectly homologized the forward extending anterior branch of the lamella characteristica as the radix ( Tu & Li, 2006b: figs 8, 27, 46). Here we have corrected this mistake and have relabelled each structure with its correct morphological term ( Fig. 7). Consequently, the structure labelled as ‘R’ in Tu & Li (2006b: fig. 8, 18, 27, 46) is relabelled here as LC 1; ‘ LC 1 ’ and ‘ LC 3 ’ in Tu & Li (2006b: figs 8, 18, 27, 46) are relabelled here as LC 3 and posterior terminal apophysis, respectively.
We document the presence of internal abdominal structures that we have interpreted to be of glandular nature (‘special glands’; see Fig. 20A, E View Figure 20 ). These structures, visible after enzymatic digestion of abdominal tissues, can be found in a pair of clusters at or near the base of the PLS. We found these structures in females of S. longqiensis and in males of S. wulingensis .
Phylogenetics: Solenysa falls within the ‘distal erigonines’ clade and its monophyly is unambiguously supported by numerous putative synapomorphies (see Discussion). The 12 Solenysa species are grouped into four clades ( Figs 5 View Figure 5 , 6 View Figure 6 ): the S. melloteei , S. longqiensis , S. protrudens , and S. wulingensis groups, each with a distinct genitalic pattern.
Natural history: Solenysa species build small webs at base of grasses, above ground. They also can be found in leaf-litter by sifting.
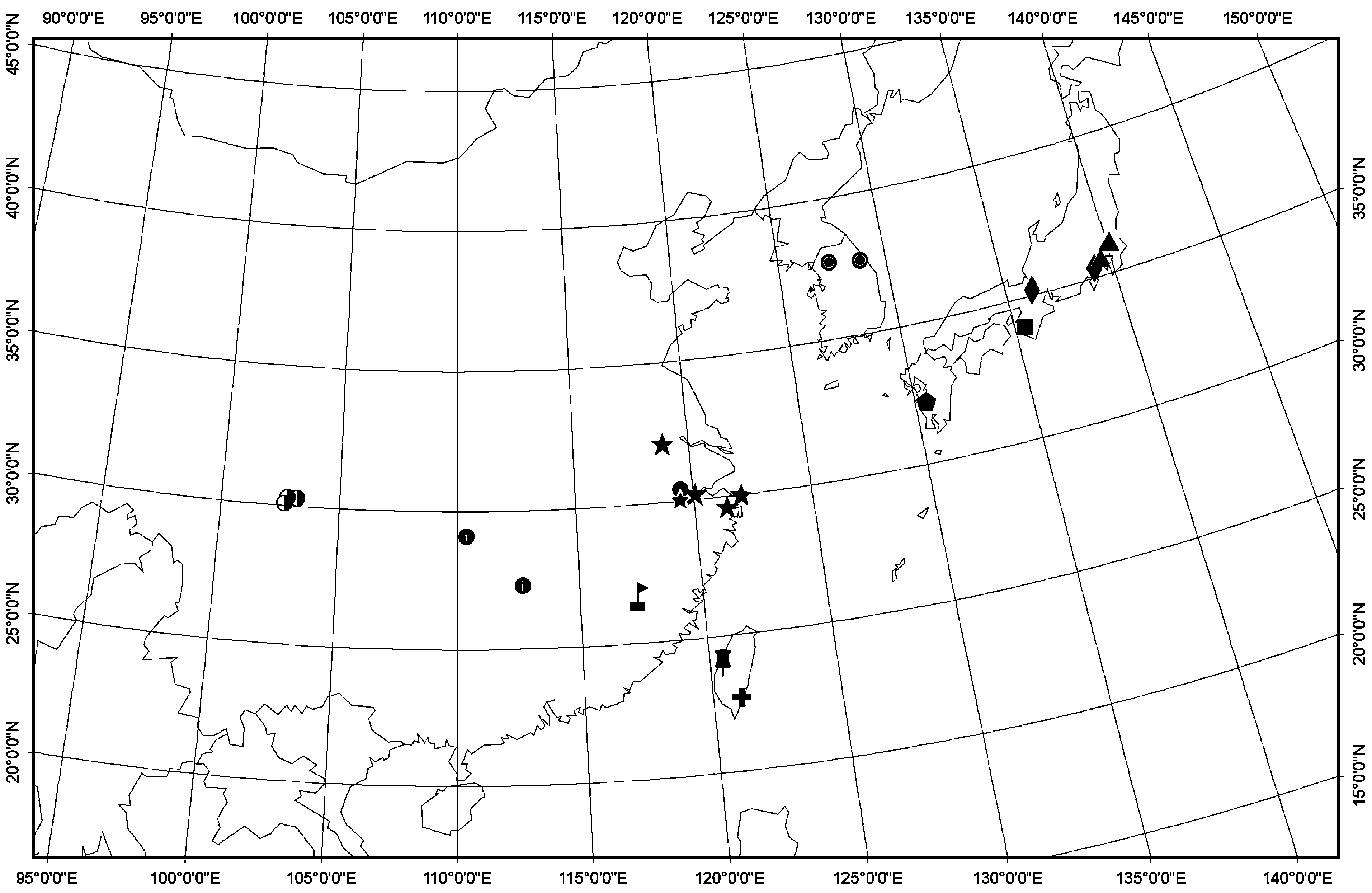
Distribution: The distribution of Solenysa species shows conspicuous regionality ( Fig. 22 View Figure 22 ). To date, all Solenysa species have been recorded from 22.70°– 37.74°N, 102.70°– 139.30°E. The S. wulingensis clade includes four species distributed in the south-east of the mainland of China and Korea Peninsula; the S. melloteei clade includes four species only found in Japan; the S. longqiensis clade and the S. protrudens clade, each including two species, are distributed on both sides of the Taiwan Channel. Every species is limited to its type locality and the adjacent area; none of them is widely distributed, but this could very well be the result of insufficient sampling.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Solenysa
| Tu, Lihong & Hormiga, Gustavo 2011 |
Solenysa Simon, 1894: 677
| Simon E 1894: 677 |