Dipolydora echinata, Radashevsky, 2022
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5162.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:11BA9195-9A8E-4098-975B-E010627F9DFD |
|
DOI |
https://doi.org/10.5281/zenodo.6817795 |
|
persistent identifier |
https://treatment.plazi.org/id/039887AA-FFBA-8C13-4D94-41AE30DBF84C |
|
treatment provided by |
Plazi |
|
scientific name |
Dipolydora echinata |
| status |
sp. nov. |
Dipolydora echinata View in CoL sp. nov.
http://zoobank.org:act: E9F81CFA-BD86-433A-A1AF-7BC235544D5D
( Figs 2 View FIGURE 2 & 3 View FIGURE 3 )
Type material. South China Sea, Vietnam, Nha Trang Bay: Dung Is., 10 m, in shell of jewel box clam Chama sp. , 19 May 2006, MIMB 42700 (paratype); Tam Is., 10 m, in fire coral Millepora platyphylla Hemprich & Ehrenberg , 22 May 2006, MIMB 42701 (2 paratypes); Dung Is., 10 m, in shell of saddle oyster Placuna ephippium (Philipsson) , 10 Jun 2006, MIMB 42702 (holotype). Complete data on material examined is provided in Supplementary Table S1 View TABLE 1 .
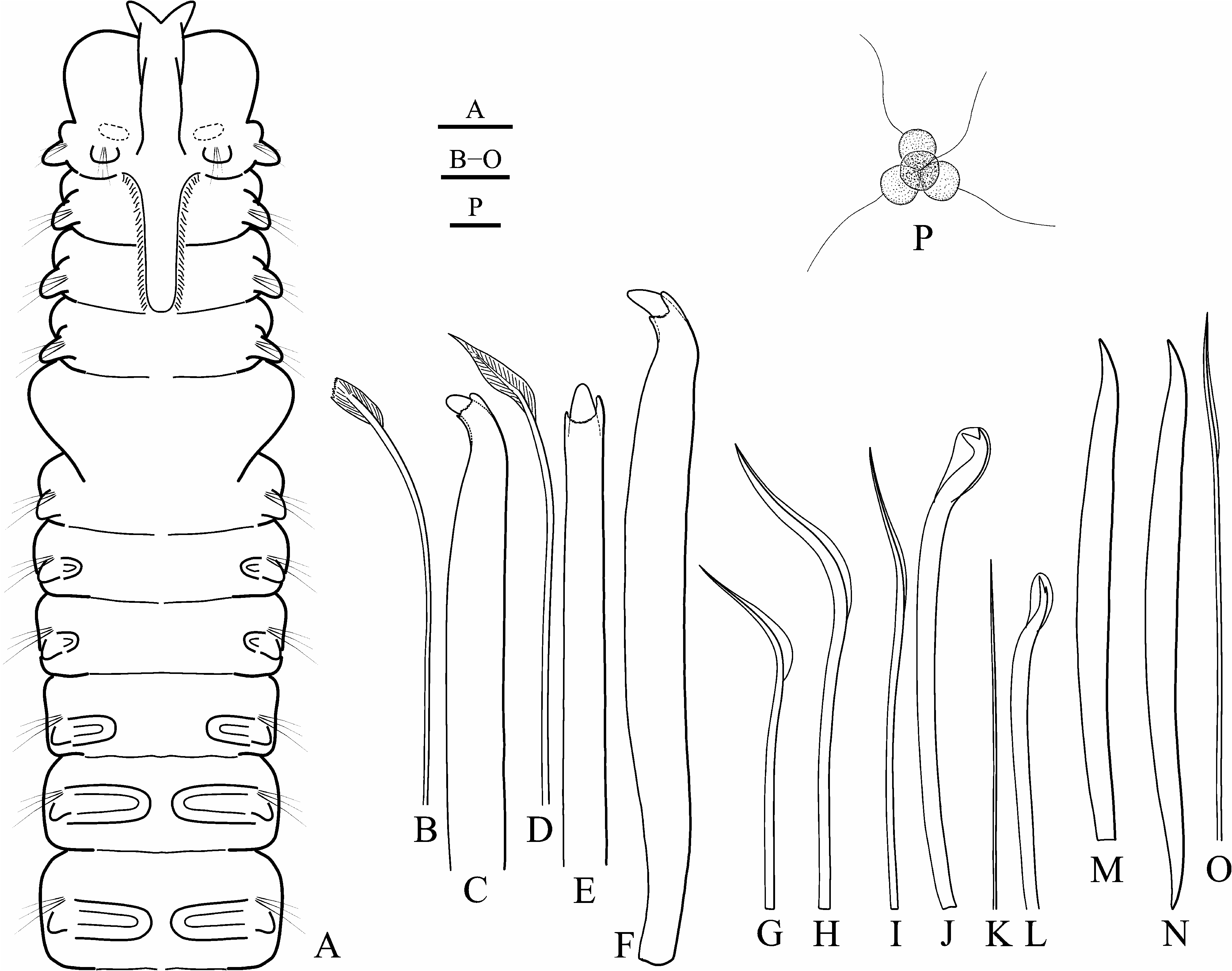
Adult morphology. Adults up to 20 mm long, 0.4 mm wide for 116 chaetigers; holotype 15 mm long, 0.3 mm wide for 113 chaetigers. Body light tan in life; distinct pigmentation absent. Prostomium bifurcated anteriorly, with shallow transverse fissure at level of chaetiger 1 separating narrow posterior caruncle extending to end of chaetiger 3 ( Fig. 2A View FIGURE 2 ); caruncle shorter in small individuals. Anterior part of caruncle, just behind fissure, inflated, but occipital antenna absent. Eyes absent. Palps as long as 15−20 chaetigers, with frontal longitudinal groove lined with short cilia, latero-frontal motile compound cilia, and short compound non-motile cilia arising directly from palp surface and scattered on lateral and abfrontal palp surfaces.
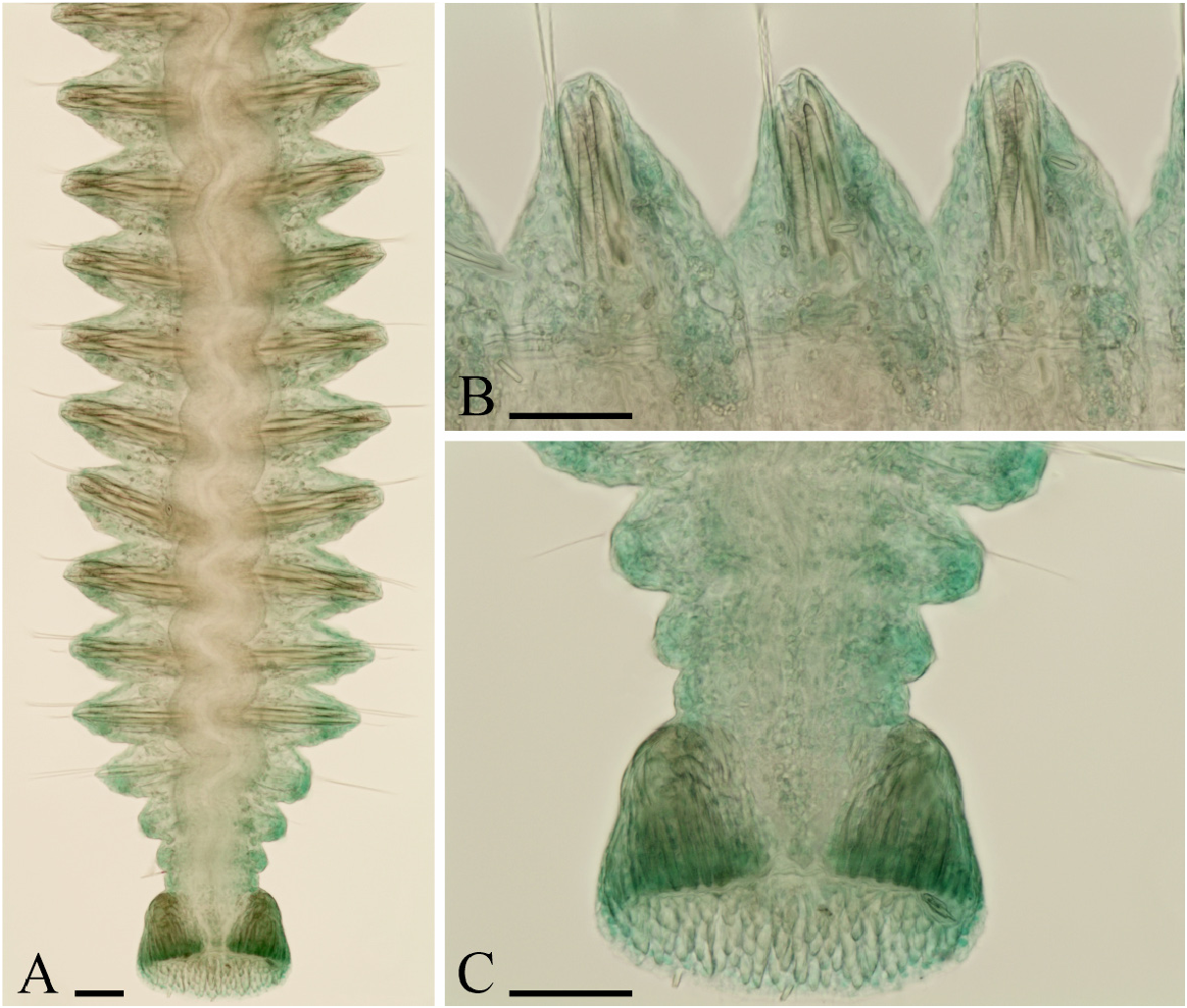
Chaetiger 1 with short capillaries and small postchaetal lamellae in both rami. Posterior notopodia (from chaetiger 36 in holotype) with up to six awl-like spines in addition to 1−5 slender capillaries in a tuft ( Figs 2 View FIGURE 2 M−O, 3A, B). In live individuals, spines entirely embedded into body; in fixed specimens, distal tips often slightly protruding out of notopodia. Distal ends of spines pointed and slightly curved. Partially developed spines with basal end truncate ( Fig. 2M View FIGURE 2 ); fully developed spines double-edged, with basal and distal ends pointed ( Fig. 2N View FIGURE 2 ).
Chaetiger 5 larger than chaetigers 4 and 6, on each side with up to four geniculate dorsal superior capillaries ( Fig. 2H View FIGURE 2 ), six heavy falcate spines alternating with bilimbate-tipped companion chaetae ( Fig. 2 View FIGURE 2 B−F) and arranged in an oblique slightly curved row, and seven ventral capillaries ( Fig. 2G View FIGURE 2 ). Dorsal superior and ventral capillaries winged, shorter than those chaetae on chaetigers 4 and 6. Falcate spines with thin transverse subdistal collar on convex and lateral sides; lateral parts of collar usually prominent, resembling small lateral teeth or spurs ( Fig. 2C, E, F View FIGURE 2 ).
Hooded hooks in neuropodia from chaetiger 7, up to seven in a series, accompanied by 1−2 inferior winged capillaries ( Fig. 2I View FIGURE 2 ) until chaetigers 8−10, and alternating with 1−2 hair-like alimbate capillaries ( Fig. 2K View FIGURE 2 ) in 10−20 posterior neuropodia. Hooks bidentate, with slightly curved shaft without constriction; upper tooth well developed, at about right angle to main fang in anterior neuropodia ( Fig. 2J View FIGURE 2 ), greatly reduced and closely applied to main fang in posterior neuropodia ( Fig. 2L View FIGURE 2 ).
Branchiae from chaetiger 7 to chaetiger 50 in 113-chaetiger holotype, to chaetiger 37 in a 85-chaetiger individual, and to chaetiger 42 in a 116-chaetiger individual; full-sized from chaetigers 11−14, free from notopodial postchaetal lamellae, flattened, with surfaces oriented parallel to body axis. Of two paratypes ( MIMB 42701 View Materials ), one individual with branchiae from chaetiger 9, and another with branchiae from chaetiger 10. In these worms, first ten anterior chaetigers slightly smaller than succeeding chaetigers, suggesting recent regeneration likely after asexual reproduction by architomy or after a partial predation event. Branchiae from chaetiger 9 or 10 also in some other examined but not fixed individuals .
Nototrochs from chaetigers 9−13, composed of single rows of ciliated cells. Intersegmental ciliation absent.
Pygidium cup-shaped, with dorsal gap to incision, white due to numerous fusiform glandular cells ( Fig. 3A, C View FIGURE 3 ).
Glandular pouches in neuropodia from chaetiger 7, full-sized from chaetigers 7−8 and diminishing in size after chaetigers 10−15.
Digestive tract with gizzard-like structure beginning from chaetigers 16−21 and extending through 1−2 chaetigers. Gizzard composed of anterior transparent muscular part and posterior white, apparently secretory, part. Rectum white in 3−5 posterior most chaetigers.
Nephridia from chaetiger 7, opening to exterior via two nephridiopores situated on lateral sides in anterior sterile chaetigers and on dorsal side in fertile chaetigers.
Habitat. Dipolydora echinata sp. nov. is an opportunistic borer, making U-shaped burrows in shells of the saddle oyster Placuna ephippium (Philipsson) , razor clam Pinna cf. bicolor Gmelin , jewel box clam Chama sp. , vase snail Vasum turbinellus (Linnaeus) , spindle snail Polygona infundibulum (Gmelin) occupied by hermit crab, in fire coral Millepora platyphylla Hemprich & Ehrenberg , and under crust of coralline algae on shells of the penguin’s wing oyster Pteria penguin (Röding) .
Reproduction. Two mature individuals (holotype and a not fixed specimen) were males with sperm from chaetiger 38 through most of the body. Spermatogonia proliferated in testes; spermatogenesis occurred in the coelomic cavity. Spermatids were joined in tetrads ( Fig. 2P View FIGURE 2 ). Spermatozoa were introsperm with elongated straight head about 1 µm in diameter, head+middlepiece 12 µm long, acrosome 3 µm, nucleus 5 µm, middlepiece 4 µm, and flagellum 38 µm long. Egg morphology and larval development of the species remain unknown.
Remarks. Dipolydora echinata sp. nov. co-occurs in shells with D. spinosa sp. nov. (see below). Both species have bifurcated prostomia, awl-like spines in the posterior notopodia, and cup-shaped pygidia with only dorsal incision. They differ in that D. echinata sp. nov. has no occipital antenna, caruncle extending to the end of chaetiger 3, branchiae beginning on chaetiger 7, awl-like spines in the posterior notopodia double-edged, up to six in a series, and spermatids joined in tetrads, while D. spinosa sp. nov. has an occipital antenna, caruncle extending to the middle of chaetiger 5, branchiae beginning on chaetigers 8−9, awl-like spines in the posterior notopodia with basal part blunt, up to three in a series, and spermatids joined in octads. Moreover, D. spinosa sp. nov. has chaetiger 5 falcate spines with spoon-like hollow on subdistal concave side and a thin transverse shelf on convex side, while spines of D. echinata sp. nov. have no spoon-like hollow on concave side and have a wide collar on convex side with lateral parts prominent, resembling small teeth or spurs.
By having a cup-shaped pygidium with only dorsal incision, D. echinata sp. nov. is similar to D. protuberata ( Blake & Kudenov, 1978) from Victoria, Australia, and D. pilikia ( Ward, 1981) from Hawaii. The species differ in that D. echinata sp. nov. has caruncle extending to the end of chaetiger 3, branchiae beginning on chaetiger 7 and lacking from the posterior half of the body, and chaetiger 5 falcate spines with transverse subdistal collar with lateral parts prominent, resembling small teeth or spurs, while D. protuberata and D. pilikia have caruncles extending beyond chaetiger 3, branchiae beginning on chaetigers 8−9 and continuing beyond the middle of the body, and chaetiger 5 falcate spines with a subterminal protuberance. Blake & Kudenov (1978: 250) and Ward (1981: 724) noted that D. protuberata and D. pilikia have “short acicular spines” in addition to capillaries in the posterior notopodia. These spines, however, were not illustrated for D. pilikia . Ward (1987: 354) also noted about D. pilikia : “This very common bioeroder in coral rock … has been collected from coral rock, coral sediment blocks, and sediment in Kaneohe Bay, from sand and rubble on the reef flat at Fort Kamehameha, and from sediment in Pearl Harbor, Oahu.” This description may be interpreted as some worms were living in tubes in soft sediments while others bored into corals. Dipolydora pilikia has never been redescribed and not reported again from Hawaii. Dipolydora protuberata was found only in tubes in soft sediments while D. echinata sp. nov. was found only in burrows in mollusc shells.
Etymology. The species name, feminine for Latin echinatus (echinate, prickly), refers to the awl-like spines in the posterior notopodia of adults.
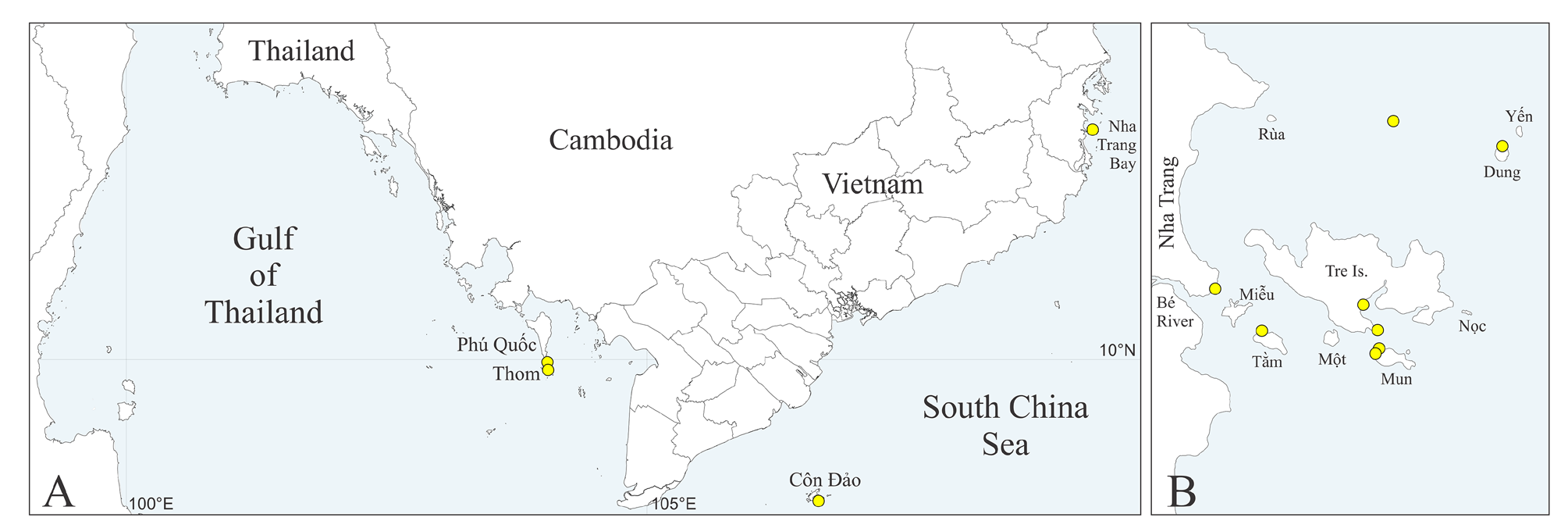
Distribution. South-China Sea, Vietnam (see Table S1 View TABLE 1 , Fig. 1A, B View FIGURE 1 ).
| MIMB |
Museum of the Institute of Marine Biology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |