Microspio moorei (Gravier, 1911)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5120.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:245EF56E-F4B3-495E-AB97-82F2125B6C30 |
|
DOI |
https://doi.org/10.5281/zenodo.6391233 |
|
persistent identifier |
https://treatment.plazi.org/id/039D8019-831C-3121-FF4B-75C3B844FD16 |
|
treatment provided by |
Plazi |
|
scientific name |
Microspio moorei (Gravier, 1911) |
| status |
|
Microspio moorei (Gravier, 1911) View in CoL
Figures 2A–G View FIGURE 2 ; 3A–K View FIGURE 3
Mesospio moorei Gravier, 1911a: 100–105 View in CoL , Plates VII, figs 80–83, VIII, 84–86.— Gravier, 1911b: 313.— Augener, 1932: 39–40.— Hartman, 1966: 17, Plate IV, figs 1–3.— Bellan, 1975: 789.— Blake, 1983: 241.— Sicinski et al. 2011: 35, Table 1: 37.
Microspio moorei Foster, 1971: 35 View in CoL .— Maciolek, 1990: 1113–1115, Table 1.
Microspio cf. moorei Petti et al. 2006: 166 View in CoL , Table 1.— Sicinski et al. 2011: 37.
Microspio sp. Barbosa et al. 2010: 1158, Table 1.
Material examined. All samples collected in Fildes Bay, King George Island, South Shetland Islands, Antarctic Peninsula; 62º12’31,32”S 58º57’45,86”W: UDEA: CEMUA: ANNE:001594 (8 specimens); 0.3 m depth, low tide. Coll. M. Londoño & I. Fonseca. Feb. 24, 2017 GoogleMaps . UDEA: CEMUA: ANNE:001595 (7); 0.3 m depth, low tide. Coll. M. Londoño & I. Fonseca. Feb. 24, 2017 . UDEA: CEMUA: ANNE:001596 (2); 1.3 m depth low tide. Coll. M. Londoño & I. Fonseca. Feb. 26, 2017 . UDEA: CEMUA: ANNE:001597 (9); 1.3 m depth low tide. Coll. M. Londoño & I. Fonseca. Feb. 24, 2017 . UDEA: CEMUA: ANNE:001598 (3); 1.3 m depth low tide. Coll. M. Londoño & I. Fonseca. Feb. 24, 2017 .
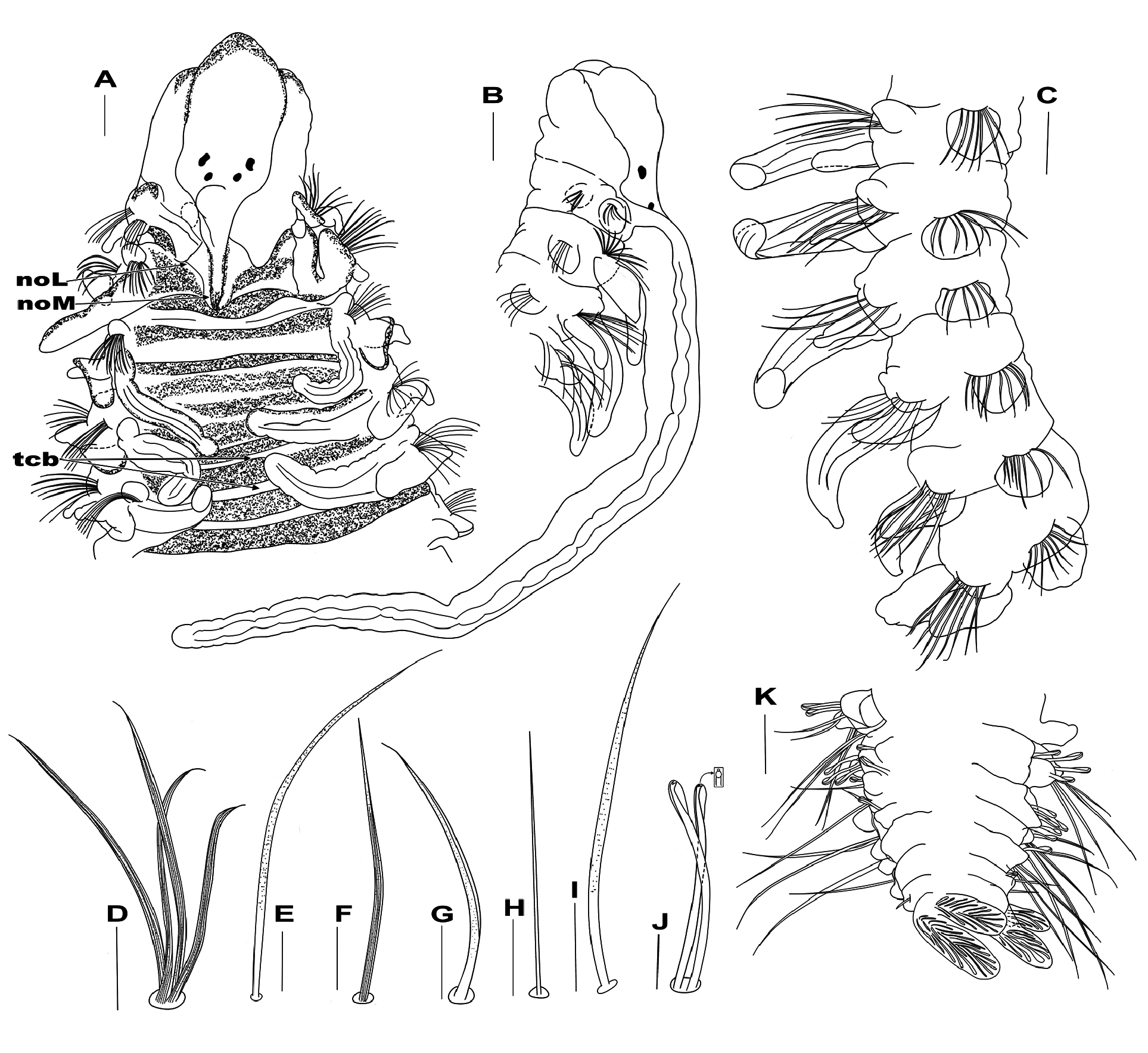
Description. Complete specimens with 4.1–12.3 mm long and 0.5–1.5 mm wide with 36–62 segments. In life and in alcohol, prostomium, peristomium, caruncle, and dorsum of first five chaetigers dark, subsequent segments with diminishing pigmentation, palps dark ( Fig. 2A, B View FIGURE 2 ), ventral surface of first five segments with dark pigmentation, subsequent segments with pigmentation decreasing gradually, concentrated along midline up to chaetiger 12 ( Fig. 2C View FIGURE 2 ); in life, specimens pink with visible blood vessel running inside the branchiae ( Fig. 2D View FIGURE 2 ); ventral epidermal glands absent.
Prostomium broadly rounded and tapered anteriorly ( Figs 2A View FIGURE 2 , 3A View FIGURE 3 ), posteriorly narrow, tapered in a narrow caruncle reaching the base of chaetiger 2 ( Fig. 3A View FIGURE 3 ), with slightly elevated keel near base of palps ( Fig. 3A View FIGURE 3 ). Occipital tentacle absent. Two pairs of black eyespots arranged in trapezoid, anterior pair larger, crescent-shaped, widely spaced; posterior pair smaller, rounded, closely spaced ( Figs 2A, B, D View FIGURE 2 , 3A View FIGURE 3 ). Peristomium long, collar-like, partially enveloping prostomium and extending around base of palps, not forming lateral wings ( Figs 2A View FIGURE 2 , 3A, B View FIGURE 3 ), separated from chaetiger 1. Palps long, thick, extending to chaetigers 8–11; palps longitudinally grooved, with dark brown pigment along both sides, except basally ( Figs 2B, D, E View FIGURE 2 , 3B View FIGURE 3 ); palpal sheath short, smooth, fused to anterior base of palps ( Fig. 2B, D View FIGURE 2 ).
Nuchal organs with medial ciliary bands around caruncle, extending to chaetiger 2, then turning laterally, with small gap between this and the second lateral band. From chaetiger 3, dorsum with two transverse rows of ciliated patches; the first row extending between branchial bases; the second row widely separated from the first, near segmental groove ( Figs 2A View FIGURE 2 , 3A View FIGURE 3 ), transverse rows of ciliated patches visible ( Fig. 2A View FIGURE 2 ) up to around chaetiger 22.
Branchiae from chaetiger 2 to almost posterior end; the first pair of branchiae slightly shorter and thinner or as long as those on following chaetigers ( Figs 2A, D View FIGURE 2 , 3A, C View FIGURE 3 ); longest through mid-body region, reaching dorsal midline ( Fig. 2A, E View FIGURE 2 ), then becoming very small; short posteriorly ( Fig. 2F View FIGURE 2 ); branchiae partly fused at the base with notopodial postchaetal lamellae anteriorly ( Figs 2A, B View FIGURE 2 , 3A View FIGURE 3 ), increasingly separate from lamellae posteriorly, flattened, robust, elongate, distally rounded ( Figs 2A, B View FIGURE 2 , 3A View FIGURE 3 ), with long cilia on inner margin.
Notopodial postchaetal lamellae triangular, short on chaetiger 1; lamellae on chaetigers 2–8 small, subtriangular with rounded ventral edge ( Figs 2A, E View FIGURE 2 , 3A, C View FIGURE 3 ); thereafter becoming oval and slightly decreasing in size throughout the body ( Fig. 2F View FIGURE 2 ). Notopodial prechaetal lamellae very short, rounded on chaetiger 1, robust, subtriangular on chaetigers 2–9 ( Figs 2A, B View FIGURE 2 , 3A, C View FIGURE 3 ); subsequent lamellae progressively decreasing in size, becoming round and smaller ( Fig. 2F View FIGURE 2 ). Neuropodial postchaetal lamellae small, triangular on chaetiger 1 ( Fig. 2E View FIGURE 2 ); subtriangular on chaetiger 2 ( Figs 2E View FIGURE 2 , 3B View FIGURE 3 ); subsequent neuropodial lamellae large, rounded, wider ( Figs 2E View FIGURE 2 , 3C View FIGURE 3 ), up to end of the body ( Fig. 2F View FIGURE 2 ). Neuropodial prechaetal lamellae absent.
Notopodial capillary chaetae on chaetiger 1 longer, thinner and alimbate, arranged in one row; capillary chaetae from chaetiger 2 arranged in two rows; both rows with slightly granulated, striated, unilimbate chaetae ( Fig. 3D View FIGURE 3 ); posterior row with very long and pointed chaetae. All chaetigers with an additional superior fascicle; anterior chaetigers with 4–7 long, granulated capillary chaetae ( Fig. 3E View FIGURE 3 ); middle chaetigers with short, thin, smooth and alimbate chaetae; posterior chaetigers with slender, smooth, long and alimbate chaetae ( Fig. 3K View FIGURE 3 ).
Neuropodial capillaries of chaetigers 1–3 arranged in one row; capillaries long, smooth, unilimbate; capillaries of subsequent chaetigers arranged in two rows, capillaries of both rows of same length, most dorsal capillaries stout, slightly granulated, striated and unilimbate ( Fig. 3F View FIGURE 3 ); capillaries of ventral region slender, slightly granulated, unilimbate ( Fig. 3G View FIGURE 3 ); inferior fascicle with 4–6 long, smooth, thin capillaries ( Fig. 3H View FIGURE 3 ) in position of sabre chaetae usually present in most anterior chaetigers, around chaetiger 13 with granulate and long sabre chaetae ( Fig. 3I View FIGURE 3 ), up to 3 per fascicle. Neuropodial hooded hooks ( Fig. 3J View FIGURE 3 ) from chaetigers 14–17; up to 11 hooks per fascicle, accompanied by granulated, unilimbate capillaries in first chaetiger with hooks, thereafter only hooks. All hooks bidentate, with small tooth above main tooth ( Fig. 3J View FIGURE 3 ). Pygidium long, with four short highly glandular digitate lobes surrounding the anal opening ( Fig. 3K View FIGURE 3 ).
Methyl Green staining pattern. Body destains fairly rapidly; stain is retained briefly on anterior-most end of body. Anterior parapodial lamellae initially stain deeply but rapidly lose the stain.
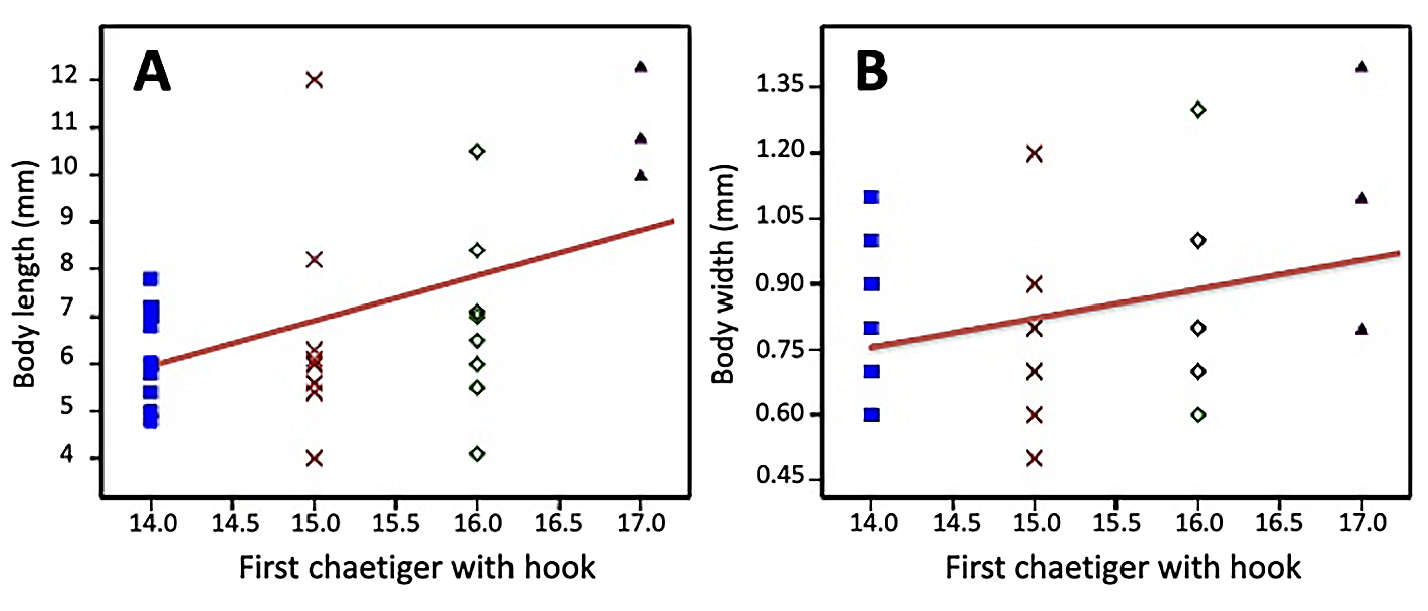
Variation. The segments where the hooded hooks started varied from segment 14 in small specimens to segment 17 in the longest specimens.
A significant positive linear regression was found between the chaetiger where the hooded hooks first appeared and the body length (R 2 = 0.22474; Permutation p = 0.0031) ( Fig. 4A View FIGURE 4 ). Specimens with an average length of 6.3 mm (SD±0.94) showed hooks starting on chaetiger 14, and as the body length increased, hooks first appeared on segments up to chaetiger 17 in individuals with a length close to 11 mm (SD±1.17).
Even though the statistical analysis was not significant (R 2 = 0.10305; Permutation p = 0.0653), it was also observed that in individuals with greater body width, hooded hooks first appeared on posterior segments, in such a way that specimens with a diameter greater than 1.1 mm (SD±0.30) had hooks starting in chaetiger 17 ( Fig. 4B View FIGURE 4 ).
A multivariate regression analysis supports these results and shows that as the polychaetes grow in length and width, the chaetiger where the hooded hooks first appear is progressively later (R 2 = 0.2235; p(regr) = 0.01907).
Discussion. Specimens herein described become the only additional material that has been used for taxonomic purpose, since none of the recent material used for ecological studies from the type locality, Admiralty Bay, and identified as Microspio moorei ( Sicinski 2004; Pabis & Sicinski 2010a, b), Microspio cf. moorei ( Petti et al. 2006) , Microspio sp. ( Barbosa et al. 2010), and Mesospio moorei ( Sicinski et al. 2011) , was available for checking their identity. Taxonomic information given by Hartman (1966) and Blake (1983) are based on the description by Gravier (1911a), and comparative notes by Maciolek & Blake (2021) are based on the description by Blake (1983). The quantity of individuals obtained from Fildes Bay, adjacent to the type locality, were sufficient to evaluate the relationship between the segment where different types of chaetae first appear and the body length and number of segments presented by each complete individual. The original description, based on the holotype with 16 mm in length, considered chaetiger 15, where hooded hooks appear for the first time, as a character with taxonomic importance ( Gravier, 1911a); nevertheless, regression analyses from additional material herein studied indicate that this character is size dependent, so increasing body length leads to hooded hooks occurring more posteriorly on the body ( Fig. 4 View FIGURE 4 ).
More analyses on ontogenetic development are needed to assess the physiological bases of this morphological variability in chaetation.
Type locality: Admiralty Bay , King George Island , South Shetland Islands, Antarctic Peninsula .
Distribution: This species has been identified only in the South Shetland Islands, Antarctic Peninsula, in Admiralty Bay by Gravier (1911a, b), Sicinski (2004), Pabis & Sicinski (2010a, 2010b), and in its different inlets (lagoons or fjord-like shaped bays), Mackellar, Martel, and Ezcurra ( Barbosa et al. 2010), in Deception Island by Augener (1932), and in Fildes Bay, King George Island, in this study. From 0.3 m (this research) to 30 m depth (sensu Augener 1932).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.