Ankersquilla pardus, Ahyong & Porter & Caldwell, 2020
|
publication ID |
https://doi.org/ 10.3853/j.2201-4349.72.2020.1758 |
|
publication LSID |
lsid:zoobank.org:pub:72036D6B-5E5E-4E76-B397-F543A09EEF32 |
|
persistent identifier |
https://treatment.plazi.org/id/0BD75B46-0194-43AD-A2E9-049982C56C18 |
|
taxon LSID |
lsid:zoobank.org:act:0BD75B46-0194-43AD-A2E9-049982C56C18 |
|
treatment provided by |
Felipe |
|
scientific name |
Ankersquilla pardus |
| status |
sp. nov. |
Ankersquilla pardus sp. nov.
http://zoobank. org/nomenclaturalActs/ 0BD75B46-0194-43AD-A2E9-049982C56C18
Figs 1–4 View Figure 1 View Figure 2 View Figure 3 View Figure 4
Holotype: UF23346 , ♂ (TL 52 mm), Moorea [ French Polynesia], NW side of Cook Bay, off Gump station, 17°29.406'S 149°49.578'W, back-reef, sandy reef flat with massive coral blocks and rubble, 1 m, deep in sand under large piece of rubble, fcn BMOO-6054, sta BIZ 5, coll. A. Anker, 17 October 2009 GoogleMaps . Paratype: AM P102286 , ♀ (TL 53 mm) , Moorea, SW coast, lagoon off Nihimaru estuary, 17°31.998'S 149°54.306'W, back-reef, sandy reef flat with massive corals, algae and rubble, deep under large algaecovered piece of rubble, 1.5 m, fcn BMOO-4918, sta MIB 167 View Materials , coll. A. Anker, 29 October 2008 .
Other material examined. AM P104060 , 1 female (TL 82 mm), Indonesia (probably Bali), purchased, aquarium trade, July 2006 .
Description. Eye subtriangular, extending almost to end of or slightly beyond antennular peduncle article 1; cornea strongly bilobed, set transversely on stalk, with 6 mid-band rows of ommatidia; CI 419–501. Ophthalmic somite anterior margin transverse; ventral surface with blunt spine arising proximally and minute distomedian granule. Ocular scales triangular, separate, anterior margins concave, apices directed laterally.
Antennular peduncle 0.58–0.71CL. Antennular somite dorsal processes directed anterolaterally, apices blunt.Antennal protopod dorsally unarmed; with small ventrodistal spine and 1 ventral papilla. Antennal scale length 0.58–0.61CL.
Rostral plate linguiform to subtriangular, slightly wider than long, widest basally, lateral margins straight, convergent, apex rounded; low, indistinct median carina. Carapace anterolateral angles bluntly angular, anterior margins straight; carinae absent except for marginal carina, indicated posterolaterally.
Mandibular palp 3-segmented. Maxillipeds 1–5 with epipod. Maxillipeds 3–4 propodi ovate, rounded, without distoventral ribbing. Maxilliped 5 basal article without ventrally directed spine.
Raptorial claw dactylus with 3 teeth; outer margin very weakly sinuous on proximal half, curving distally, with distinct basal notch. Propodus with 3 movable spines proximally, distal margin unarmed; distal margin unarmed; propodus shorter than carapace, when folded, not extending posterior beyond merus; PI 105 (male), 109–120 (female). Carpus dorsal margin terminating in short, ventrally directed spine. Merus inferodistal margin rounded, unarmed. Ischium shorter than one-fourth merus length. Basis lateral surface with 2 or 3 short denticles.
Pereopods 1–3 proximal-most article with outer ventrolaterally directed triangular lobe; inner margin unarmed. Endopod distal article slender, liguliform, tapering distally; outer and inner distal margins setose.
Thoracic somite 5 lateral process obsolete, with small ventrally directed spine. Thoracic somites 6–8 lateral process rounded to subtruncate; faintly indicated lateral carina. Thoracic somite 6 female gonopore with bilobed median papillae flanked by lower lateral papilla. Thoracic somite 8 sternal keel low, rounded.
Male pleopod 1 endopod with lateral lobe on distal ‘endite’.
Abdominal somites loosely articulated; somites 1–4 smooth dorsally; posterior margin unarmed; 3 shallow grooves laterally (upper two corresponding to intermediate and lateral carina) and marginal carina; somites 1–3 posterolaterally unarmed; somite 4 with posterolateral spine; ventral pleural margin straight to faintly concave. Abdominal somite 5 with posterior half to two-thirds covered with short posteriorly directed spines; posterior margin lined with posteriorly directed spines; laterally with 3 posteriorly armed carinae (first and third corresponding to intermediate and lateral carinae) and posteriorly armed marginal carina; ventral pleural margin distinctly concave. Abdominal somite 6 surface entirely covered with short posteriorly directed spines; posterior margin lined with posteriorly directed spines; lateral carina indicated, lined with spines; 2 triangular spines anterior to uropodal articulation, apices simple or bifid (in largest specimen); sternum posterior margin unarmed medially, with 3–10 small spines on each posterolateral margin.
Telson length half width, dorsal outline evenly curved; dorsal surface and posterior margin densely covered with short, curved spines; median carina indicated by longitudinal row of short, curved spines of similar size to adjacent spines on telson surface; submedian teeth short, margins spinose, movable apices conical, curved, separated by narrow, U-shaped cleft; submedian denticles absent; with single spiniform submarginal intermediate and lateral denticles, dorsal lobe absent. Intermediate and lateral teeth short, stout, spinose, not produced beyond general posterior telson outline. Ventral surface covered with short spines; postanal carina absent.
Uropodal protopod dorsal surface covered with short spines; anterior margin convex, unarmed; inner primary spine ventrally carinate, distinctly longer than outer spine; inner margin with 3–5 graded spines; outer margin with 4–11 short spines, unarmed anterior to endopod articulation. Uropodal exopod proximal segment dorsal surface with patch of short spines on inner half; inner margin concave, unarmed; slender, curved distoventral spine; outer margin with 7 or 8 graded movable spines, distalmost not reaching beyond midlength of distal segment. Exopod distal segment longer than proximal segment; dorsal midrib with 7–11 short spines; ventral midrib with 0–3 minute spines. Endopod elongate, reniform, apex bluntly rounded; length 4.57–4.67 × width; dorsolateral surface with row of 10–15 short spines.
Colour in life ( Figs 2 View Figure 2 , 3 View Figure 3 ). Overall pale yellowish-tan with diffuse whitish mottling and numerous black or black-brown spots over cephalothorax, abdomen, tailfan and pereopods, forming leopard-spotted pattern. Eyes pale yellow-tan; cornea silver.Antennular and antennal peduncles with diffuse white speckling; antennular articles distally yellow-brown. Antennal protopod pale with black-brown spots; scale speckled white with dark, irregular patch slightly proximal to midlength and at distal end. Raptorial claw propodus and carpus translucent white and diffuse irregular brown mottling and diffuse blue-green highlights; movable propodal spines translucent white overall (TL 52–53 mm) to black-green on proximal two-thirds (TL 82 mm); dactylus translucent white teeth margined with orange-brown; ischiomerus pale yellowish-tan with diffuse white mottling and several dark diffuse spots, distal margin diffuse blue-green. Uropodal exopod distal article and endopod distal half dark-brown; marginal setae dull-pink.
Etymology. Derived from the formal name of the Leopard, Panthera pardus ( Linnaeus, 1758) , for the distinctive, leopard- spotted colour pattern of the new species; used as a noun in apposition.
Measurements. Male (n = 1) TL 52 mm; female (n = 2) TL 53–82 mm. Other measurements of holotype: CL 9.0 mm, antennular peduncle length 6.4 mm, antennal scale 5.2 mm, propodus length 8.3 mm, abdominal somite 5 width 10.3 mm.
Habitat. The French Polynesian specimens were collected from shallow (1–1.5 m) sandy back-reef sites with rubble and algae; both were burrowed beneath coral boulders. The Marshall Islands individual was photographed at 8 m depth at Kwajalein Atoll in a lagoon patch of Halimeda sp. on sand. The precise collecting locality of the TL 82 mm Indonesian specimen is not known, but in 2000, RLC and Mark Erdmann observed but failed to capture another Indonesian individual in Tolitoli Bay, Sulawesi, dwelling in a large worm tube in massive coral head at 2 m depth.
Distribution. Central to western Pacific, from French Polynesia, the Marshall Islands and Indonesia.
Discussion
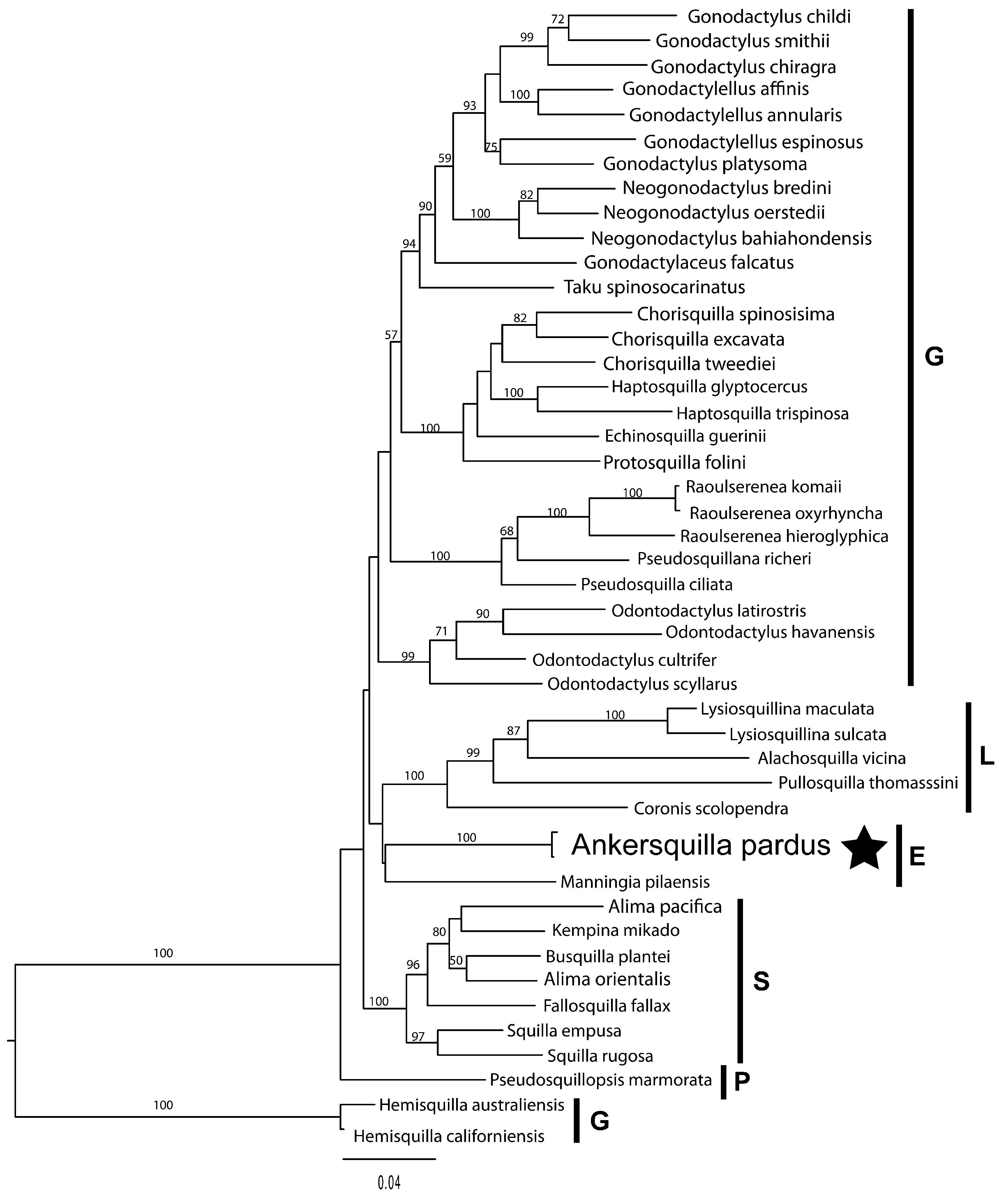
Ankersquilla gen. nov., represented by A. pardus sp. nov., is unique in Eurysquilloidea in having three teeth on the dactylus of the raptorial claw; all other eurysquillids have four ( Manningia Serène, 1962 ; Coronidopsis Hansen, 1926 ) or more dactylar teeth ( Eurysquilla Manning, 1963 ; Eurysquilloides Manning, 1963 ; Raysquilla Ahyong, 2000 ; Sinosquilla Liu & Wang, 1978 ) ( Ahyong, 1997b, 2001). Three spearing teeth on the dactylus of the raptorial claw are otherwise present only in members of the Parasquilloidea and in Pseudosquillidae Manning, 1977 (Gonodactyloidea) . Another unusual feature of Ankersquilla is the absence of upright rounded lobes associated with the intermediate and lateral denticles of the telson; these lobes are present in all other eurysquillids except for Eurysquilloides Manning, 1963 ( Ahyong & Harling, 2000; Ahyong, 2001). Perhaps the most remarkable aspect of Ankersquilla is its resemblance to some members of the Coronididae Manning, 1980 , in the superfamily Lysiosquilloidea Giesbrecht, 1910 . The uniformly and densely spinose surface of abdominal somite 6 and the telson of Ankersquilla , with a wide, semi-circular telson having short primary teeth resemble the condition in the coronidid genus, Neocoronida Manning, 1976 . This resemblance between the two genera is further accentuated by the simple, unarmed rostral plate, wide ocular scales, subtriangular eyes and strikingly similar uropod structure (compare Fig. 1M, P View Figure 1 with Manning, 1972: fig. 1e, g). Although Ankersquilla is clearly not a lysiosquilloid, as indicated by the simple and ovate, rather than ribbed, quadrate propodi of maxillipeds 3–4, and eurysquilloidform male pleopod 1 endopod ( Ahyong & Harling, 2000), it should not be assumed that the similarities to Neocoronida are the result of convergence. The maxilliped 3–4 propodi of Neocoronida also lack ventral ribbing ( Manning, 1976; Adkison et al., 1983) and the intermediate and lateral denticles of the telson are ventrally recessed, as in eurysquilloids. Thus, Neocoronida could instead prove to be a eurysquilloid rather than lysiosquilloid, but further assessment of other members of the Coronididae are required prior to making formal changes to the classification. Also, of possible phylogenetic significance is that the two eurysquilloid exemplars form a clade that is placed closer to the lysiosquilloids ( Fig. 4 View Figure 4 ) than to the squilloids and parasquilloids as indicated by previous analyses ( Ahyong & Harling, 2000; Van Der Wal et al., 2017). Nodal support for the eurysquilloid-lysiosquilloid affinity is low (as are the relationships between other superfamilies), but such a relationship, if corroborated, would indicate that the dorsoventrally flattened and generally loosely articulated body form shared by most members of Eurysquilloidea and Lysiosquilloidea could be synapomorphic rather than convergent.
Most eurysquilloids have variously spinose posterior abdominal somites and telson, but the surface sculpture and telson outline is not usually obscured as it is in Ankersquilla . Also, the median carina of the telson in Ankersquilla , which is distinct in most eurysquilloids, is instead indicated only by a longitudinal row of spines of similar size to the surrounding telson spines. Among eurysquilloids, similarly unusual abdominal and telson ornamentation is approached only in Sinosquilla . Both species of Sinosquilla have dense dorsal spination on abdominal somite 6 and the telson, which largely obscures surface sculpture ( Ahyong, 2001: fig. 16; 2010: fig. 1C, D). In Sinosquilla sinica Liu & Wang, 1978 , the median carina of the telson is distinct and unbroken, but in S. hispida Liu & Wang, 1978 , the overall dorsal spination is more uniform (albeit more pronounced) and the median carina of the telson is indicated by a row of spines, much like that of A. pardus . Ankersquilla and Sinosquilla , however, are otherwise dissimilar and probably not closely related, being readily separated by numerous features including: three teeth on the dactylus of the raptorial claw in Ankersquilla (eight or more in Sinosquilla ); a short, rounded rostral plate in Ankersquilla (long, spiniform in Sinosquilla ); intermediate and lateral telson denticles without upright lobes in Ankersquilla (lobes present in Sinosquilla ); and short, spiniform intermediate and lateral primary telson teeth that in Ankersquilla do not extend beyond the general dorsal outline (prominent, lobe-like in Sinosquilla ). The phylogenetic position of Ankersquilla within the eurysquilloids is presently unclear and awaits comprehensive revision and analysis of all genera (currently underway).
The Indonesian specimen of A. pardus (TL 82 mm; AM P104060; Fig. 2A,C View Figure 2 ) survived in captivity for approximately six and one half years during which it regularly moulted but remained essentially the same size, suggesting that it had already attained maximum length. In captivity this animal fed on shrimp and crabs, but did not break open snails and hermit crabs. Despite their wide geographic separation, the Indonesian specimen of A. pardus agrees closely with the smaller French Polynesian type specimens (TL 52–53 mm), differing chiefly in the more tapering rostral plate, blunter dorsal telson and abdominal spines, more numerous short spines overall (most notably those of abdominal somite 5 covering the posterior two-thirds instead of half), and slightly greater spination along the lateral margin of the uropodal protopod (11 versus 4–8) and posterolateral margins of the abdominal sternite 6 (7–10 versus 3–5). These differences are probably a function of the much larger size of the Indonesian specimen (TL 82 mm versus TL 52–53 mm). Evidently, A. pardus has strong dispersal capabilities given the low (1%) COI sequence divergence between Indonesian and French Polynesian specimens.
The distinctive coloration of A. pardus is consistent in all of the specimens examined, including the individual observed but not captured in Tolitoli Bay, Indonesia (see Habitat above). We identify an individual from the Marshall Islands (photographed but not captured; Fig. 3 View Figure 3 ) as A. pardus based on the visible morphology and the distinctive colour pattern. Although common names are seldom used for stomatopods, we here propose Leopard Mantis Shrimp for A. pardus , given its distinctive colour pattern.
ACKNOWLEDGMENTS. Thanks go to Gustav Paulay for the loan of material, Arthur Anker and Mark Erdmann for their efforts in the field, and Scott Johnson for alerting us to the presence of A. pardus in the Marshall Islands and for permission to use Fig. 3 View Figure 3 . We gratefully acknowledge the French-US Moorea Biocode Project (http://biocode.swala.org/about), co-partnered by IRD en Polynésie française and the University of Florida, with other partners, under which the French Polynesian specimens were collected. Thanks are also due to two anonymous reviewers for their constructive comments on the manuscript.
| AM |
Australian Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |