Guestphalinus elephantinus Fend & Rodriguez, 2017
|
publication ID |
https://doi.org/ 10.5852/ejt.2017.361 |
|
publication LSID |
lsid:zoobank.org:pub:F61CB5C7-B22E-4FAB-997A-BF99C7828C77 |
|
DOI |
https://doi.org/10.5281/zenodo.3851838 |
|
persistent identifier |
https://treatment.plazi.org/id/A30D385C-06C9-438E-AAFC-1F145905B74F |
|
taxon LSID |
lsid:zoobank.org:act:A30D385C-06C9-438E-AAFC-1F145905B74F |
|
treatment provided by |
Carolina |
|
scientific name |
Guestphalinus elephantinus Fend & Rodriguez |
| status |
sp. nov. |
Guestphalinus elephantinus Fend & Rodriguez View in CoL sp. nov.
urn:lsid:zoobank.org:act:A30D385C-06C9-438E-AAFC-1F145905B74F
Figs 10–12 View Fig View Fig View Fig
“ Guestphalinus View in CoL sp. nov.” – Zhou et al. 2010: 381, figs 1–6 (sequenced, but not morphologically described; voucher SMNH 105628).
Etymology
Based on the fanciful and homoplasious similarity to an elephant (proboscis and anteriorly-directed “tusks”).
Material examined
Holotype
UNITED STATES OF AMERICA: a dissected specimen, slide-mounted in Canada balsam, Washington, Jefferson County, Shale Creek at Clearwater Creek Road , 4 Jun. 2003, S. Fend leg. ( USNM 1422286 View Materials ). Paratypes (all from the Clearwater River drainage, on Olympic Peninsula, Washington)
UNITED STATES OF AMERICA: 1 dissected (post-reproductive), Jefferson County, Clearwater River at Upper Clearwater Camp, 27 Apr. 2004 ( USNM 1422287); 1 dissected (post-reproductive), same data as preceding ( CASIZ 220932); 1 dissected (post-reproductive), Hurst Creek near Clearwater River, 25 Apr. 2004 CASIZ 220933); 1 whole mount, immature but with gonads (DNA voucher), from type locality, 4 Jun. 2003 ( CASIZ 220934); 1 dissected (post-reproductive), Clearwater River at Upper Clearwater Camp, 27 Apr. 2004 ( MNCN 16.03/3098); 2 partially-mature whole mounts on 1 slide, Shale Creek at Clearwater Creek Road, 4 Jun. 2003 ( MNCN 16.03/3099).
Additional material (typical specimens)
UNITED STATES OF AMERICA: WASHINGTON, Jefferson County (all from the Clearwater River drainage, on Olympic Peninsula): 2 dissected (mature, reproductive organs slightly resorbed), Bull Creek near Clearwater River, 2 Apr. 2016; 2 whole mounts (immature, with small gonads), Clearwater River at Upper Clearwater Camp, 4 Jun. 2003; 4 dissected, (immature or post-reproductive), same locality as preceding, 27 Apr. 2004; 1 dissected and 16 whole mounts, several in alcohol (all immature or partially mature), Clearwater River at Copper Mine Bottom Camp, 25 Apr. 2004; 8 whole mounts (immature or partially mature), Shale Creek at Clearwater Creek Road, 4 Jun. 2003; 4 whole mounts (immature), same locality as preceding, 14 Apr. 2010; 5 whole mounts (immature), Hurst Creek, 25 Apr. 2004.
Other sites
UNITED STATES OF AMERICA: WASHINGTON: 1 dissected (slightly post-reproductive), 2 whole mounts (post-reproductive), Grays Harbor County, Middle Fork Satsop River, 27 Apr. 2004, S. Fend leg.; 2 whole mounts (1 nearly mature), Columbia County, Tucannon River below Turner Road, 29 Jul. 2011, Uttam Rai leg. – OREGON: 1 sagittal section, 1 transverse section, 3 dissected, 6 whole mounts (dissected and sectioned nearly mature, others with gonads),Yamhill County, spring at Peavine Ridge near McMinnville, 5 Dec. 1999, S. Fend leg.; 1 whole mount (immature, with gonads), Curry County, Mule Creek at upstream bridge, near Rogue River, 7 Jun. 2003, S. Fend leg.; 1 dissected (immature, with gonads), Curry County, Euchre Creek near mouth, 20 May 2013, S. Fend leg. – CALIFORNIA: 1 whole mount (immature), Santa Clara County, Alamitos Creek above Almaden Reservoir, 1 May 1997, S. Fend leg.; 1 whole mount (immature), Santa Clara County, Guadalupe Creek above Guadalupe Reservoir, below Rincon Creek, 3 Apr. 2001, S. Fend leg.; 1 dissected (mature) and 2 whole mounts (partially mature), same locality as preceding, 25 Mar. 2007, S. Fend leg.; 1 whole mount (immature), same locality as preceding, 10 Feb. 2008, S. Fend leg.; 1 whole mount (immature), same locality as preceding, 5 Dec. 2009, S. Fend leg.; 1 dissected (post-mature), same locality as preceding, 20 Jun. 2011, S. Fend leg.
Molecular data
COI, 28S and 16S sequences are based on specimens from Shale Creek and Clearwater River, Washington (details in Table 1 View Table 1 ). An additional ITS sequence (GenBank acc. no. GU592364 View Materials ) corresponds to the Clearwater River voucher SMNH 105628. A topotypic voucher is included in the type series (see above). Both COI sequences for G. elephantinus sp. nov. are identical.
Description
Specimens from the Clearwater River drainage, Olympic Peninsula, Washington
Description of somatic characters is based on mature or partially-mature (with gonads) worms from Clearwater River, Hurst Creek, Shale Creek and Bull Creek. Reproductive organs are described from the only fully-mature specimen (the holotype), from Shale Creek, and supplemented with 9 postreproductive specimens with partially-resorbed organs from nearby sites
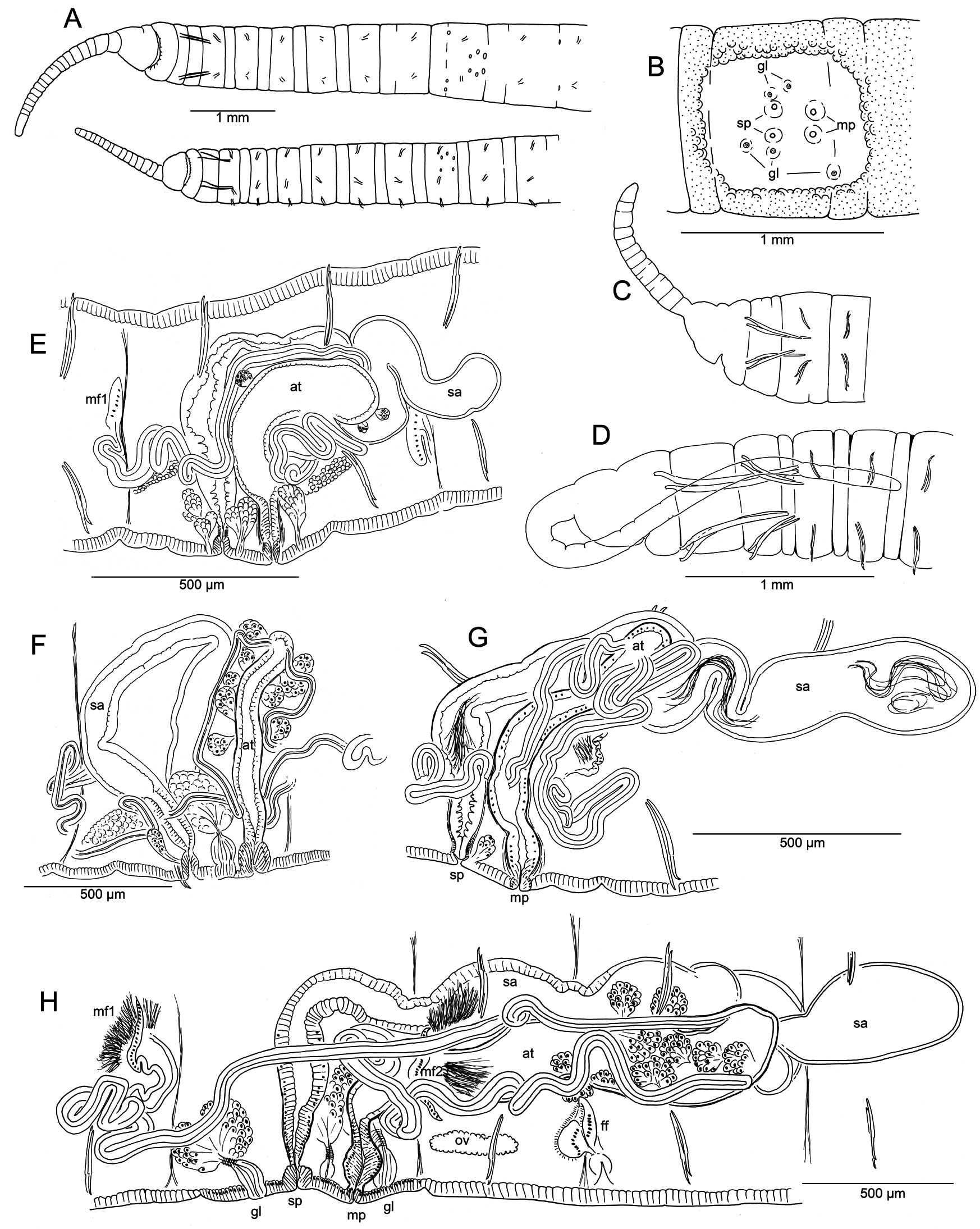
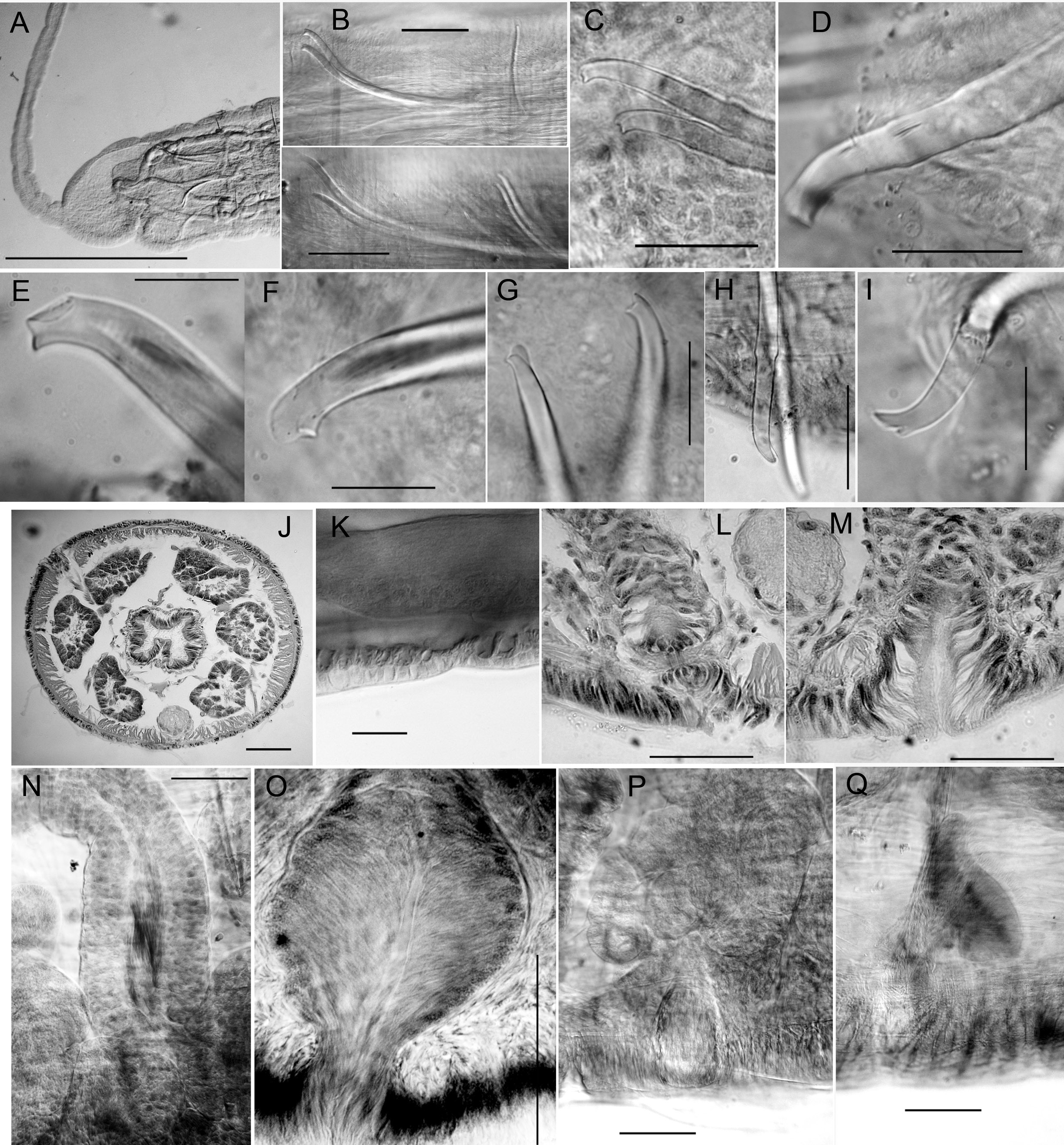
Body form and segmentation as described for G. exilis sp. nov. (see above), measurements given in Table 3. Length of proboscis 0.9–2.6 mm ( Figs 11A View Fig , 12A View Fig ), clitellum from VIII to XII in the mature worm. Chaetae in II much larger and thicker than those in posterior segments ( Table 3), bifid, with a short, thick lower tooth and a larger, rounded and laterally compressed upper tooth ( Figs10 View Fig A–C, 12C), directed anteriorly, with proximal ends protruding into posterior segments (cf. Figs 11 View Fig C–D, 12B). Dorsal chaetae in II about 30–40% longer than those in ventral bundles ( Table 3); within each bundle in II, the lateral (i.e., outer) chaeta is slightly longer, with a more distal nodulus (0.15 from tip, vs 0.24 in the median (inner) chaeta), and usually has a distal thickening that appears as a “secondary nodulus” between nodulus and tip ( Fig. 12 View Fig C–D). Chaetae in III simple-pointed or slightly bifid, and oriented more perpendicularly to body, but still facing anteriad ( Figs 10C View Fig , 12B View Fig ); much shorter than chaetae in II and slightly shorter than those in other segments ( Table 3). Chaetae in IV and posterior segments usually oriented posteriorly, simple-pointed, sigmoid ( Fig. 12H View Fig ). From III to about XX, nodulus about 0.3–0.4 from the tip, but the median (inner) chaeta in each bundle is slightly longer. In posterior segments, chaetae of similar length, but slightly thicker, and nodulus 0.25–0.35 from the tip; simple-pointed or with a small notch or dorsal keel in tail segments. Ventral chaetae absent in IX in mature or post-mature worms.
Epidermis in anterior segments 15–20 µm thick; in clitellum to 48 µm in the holotype; narrower, densely packed cells up to 30 µm on ventral side of IX (in area surrounding genital pores) in the holotype and in the post-reproductive worms; posteriorly 10–12 μm. Body-wall musculature, brain, pharynx, pharyngeal glands, nephridia, blood vessels and chloragogen as described above for G. exilis sp. nov., except that pharyngeal glands in G. elephantinus sp. nov. extend to VII in all specimens observed.
Male and spermathecal pores paired in IX. Male pores just anterior to the posterior intersegmental groove ( Fig. 11A View Fig ), slightly inside the line of ventral chaetae; spermathecal pores aligned with and in front of male pores, between or slightly posterior to chaetal bundles. Female pores paired, on chaetal line at 10/11; female funnel up to 130 µm high ( Fig. 12Q View Fig ). A variable number of copulatory glands with internal structure as described for G. exilis sp. nov.; with round secretory openings located close to spermathecal and male pores. The mature worm (holotype) has 3 pairs of petiolate copulatory glands in IX, 100–140 µm high, lateral to, in front of, and behind genital pores ( Fig. 11E View Fig ), although not all of these were visible externally ( Fig. 11A View Fig ). Copulatory glands smaller and indistinct or absent in the postreproductive worms (male duct and spermathecae expanded; large but nearly empty sperm and egg sacs extending to XV), but faint external secretory openings visible in one specimen.
Spermathecae up to 1700 µm long in the holotype; ectal ¼ is duct-like, nearly tubular (about 90 µm in diameter); ental ampulla-like portion irregular, up to about 100 µm in diameter, folded within X or extending into XI. Ectal part with irregularly columnar cells up to 35 µm high, epithelium gradually becoming thinner in ental ampulla. Spermathecae end in a short, narrow constriction surrounded by a ring of muscle fibers ( Fig. 11E View Fig , cf. Fig. 12L View Fig ) which opens in a shallow epidermal infolding, about 40 μm deep. Spermathecae of a paratype similar ( Fig. 11G View Fig ), 1200–2600 µm long, with loose sperm; ampulla thin-walled, ental diameter up to 230 µm, extending into X or as far as XII.
Anterior pair of male funnels on 8/9; posterior male funnels on 9/10, directed back into X; male funnels of post-reproductive worms indistinct, without or with small amount of sperm. Anterior vas deferens extends into VIII ( Fig. 11E View Fig ), forming a compact, convoluted mass, then penetrates 8/9, running along ventral body wall to near the male pore, then follows the atrium before joining the atrial wall and entering the lumen near the apex (junction not clear on holotype). Length of anterior vas deferens up to about 1500 μm, width of both anterior and posterior vasa deferentia 30–36 μm in the holotype. Posterior vas deferens forms a compact, convoluted mass in posterior part of IX, then follows the atrium externally before joining it near the ental end. Atria of holotype extend back into X; ectal duct (length 160 μm, width near midpoint 30–40 µm) has a thick, columnar epithelium and thin muscle coat; ectal part of duct expanded to 50 µm, with epithelial cells directed outward. Entally, there is an abrupt transition to the sacciform atrial ampulla; ampulla length up to 550 μm, width up to 130 μm, with thin-walled epithelium and a wide lumen ( Fig. 11E View Fig ). Small clusters of prostatic cells, 20–40 µm high, very sparse. Atria tubular, with ampulla not expanded in a paratype (apparently at a more advanced stage of maturity, after copulation, Fig. 11G View Fig ); length 400–800 µm, diameter 60–80 µm; prostates very small or absent; atrial duct and male pore as in the holotype.
Specimens from other localities
Satsop River, western Washington: Modified chaetae in both II and III; chaetae in II very long ( Table 3); lateral (outer) chaeta in the largest specimen with secondary nodulus, as in topotypic specimens ( Fig. 12D View Fig ). Chaetae in III smaller, but bifid and larger than chaetae in the next segments, and directed anteriad ( Fig. 11D View Fig ).
Tucannon River, eastern Washington: Typically modified chaetae in II, but without secondary nodulus; small, simple-pointed chaetae in III; ventral chaetae in posterior segments enlarged, longer and thicker than corresponding dorsals or ventrals in anterior segments posterior to III; some posterior chaetae are slightly keeled ( Fig. 10E View Fig ). In the unmated, nearly-mature worm, tubular spermatheca, vasa deferentia, atrium and prostates similar to those of the Peavine Ridge material (cf. Fig. 11F View Fig ). Copulatory glands large, up to about 150 μm high, anterolateral and posterolateral to the male pores.
Peavine Ridge, northern Oregon: Chaetae in II enlarged and modified as above, without secondary nodulus ( Figs 10H View Fig , 11C View Fig ); chaetae in III simple-pointed, smaller than other anterior chaetae. Posterior segments with ventral chaetae larger than dorsals; both dorsal and ventral chaetae slightly bifid or keeled ( Fig. 12I View Fig ). In unmated, nearly-mature worms, there is a variable number (2–4) of well-developed, petiolate copulatory glands with distinct external secretory openings, total height up to 155–290 µm ( Fig.11F View Fig ). Copulatory glands usually present between spermathecal and male pores (but lateral to both), other glands may be present anterior, lateral, or posterior to genital pores. Much smaller glands may insert at male and spermathecal pores. Atria and spermathecae both tubular, without sperm; male and spermathecal pores ( Fig. 12 View Fig L–M) as described above.
Coastal ranges, central California and southern Oregon: Highly modified chaetae in II as in typical specimens, but without secondary nodulus; the two specimens with the largest chaetae are from this region ( Table 3, Fig. 10F, I View Fig ). Chaetae in III also modified, shorter than those in II but similar in general form (bifid, with rounded, laterally flattened upper tooth; nodulus distal); longer than other chaetae in anterior segments ( Fig. 12B View Fig , F–H). Posterior chaetae usually simple-pointed, ventral chaetae keeled in tail segments (posterior to XC) of some specimens; ventrals in posterior segments larger than those in anterior segments, and larger than corresponding dorsals in some specimens.
Reproductive organs in the mature worm from Guadalupe Creek include the openings of 5 copulatory glands visible externally in IX, in ventral field, but lateral to male and spermathecal pores ( Fig. 11B, H View Fig ). Copulatory glands up to 250 µm high; extensions of granular, petiolate cells constricted by a muscular ring, terminating in a small, external secretory opening ( Fig. 12P View Fig ). Clitellum from ¼ VIII through XIII. Ectal, duct-like portion of spermatheca ( Figs 11H View Fig , 12N View Fig ) weakly differentiated from ampulla; extending to XII, thin-walled and expanded entally. Vasa deferentia as described above ( Fig. 11H View Fig ), 50–60 µm wide, joining atrium subapically; histologically similar throughout, with thick, ciliated epithelium. Atria petiolate; well-defined ectal duct; ectal part of atrial duct expanded, bulbous (up to 130 µm wide), with epithelial cells directed outward, possibly indicating a “ type 2” penis ( Fig. 12O View Fig ). Sacciform atrial ampulla extending to 11/12, with thin-walled epithelium and a wide lumen ( Fig. 11H View Fig ); petiolate prostate glands sparsely covering ampulla.
Remarks
All populations here attributed to G. elephantinus sp. nov. have highly modified bifid chaetae in II (or II and III), and their morphology differs from chaetae in all other lumbriculids. These anterior chaetae are distinctly enlarged, very weakly sigmoid, and anteriorly directed with a characteristic short, broad proximal tooth and a laterally-flattened distal tooth. In comparison, typical G. exilis sp. nov. have similar but only slightly modified (blunt-tipped or slightly notched) chaetae in II. Additional interpopulation variation in chaetal morphology suggests the existence of a species complex, but the rarity of mature specimens from most localities makes it difficult to compare populations with the usual morphological criteria, which focus on reproductive structures. Although our limited DNA sampling strongly supports species status for G. elephantinus sp. nov. and G. exilis sp. nov. morphotypes (see below), further molecular studies will be necessary to resolve the diversity of this genus in a region that appears to have extensive radiation within other lumbriculid genera ( McKey-Fender & Fender 2001; Fend & Rodriguez 2003).
The morphology of modified chaetae in segment II varies among collection sites, but appears consistent within large series of immature worms collected at some of these sites. Increased chaetal size has been associated with high water conductivity in the Naididae ( Loden & Harman 1980, for Pristina aequiseta Bourne, 1891 , or for Tubifex tubifex var. grandiseta Rodriguez, 1986 ). This does not appear to be the case for G. elephantinus sp. nov., since populations with very enlarged chaetae inhabit streams with a wide range of conductivity (<100 to>400 µS cm-1). The other western Nearctic species, G. exilis sp. nov., with much less-enlarged anterior chaetae, was collected from streams with intermediate conductivity values (ca 200–300 µS cm-1, see G. exilis sp. nov. habitat notes, above).
The reproductive organs of G. exilis sp. nov. and G. elephantinus sp. nov. appear similar, although the morphology of these structures is difficult to define, as it varies with stage of development. Although atrial morphology is commonly used in defining lumbriculid species, it has been shown that it can vary considerably over the reproductive period in Stylodrilus mollis Timm, 1998 and Trichodrilus seirei Timm, 1979 . The thin-walled, sacciform atrial ampulla in some mated specimens of both Nearctic species of Guestphalinus appears to be an unusual character for the Lumbriculidae . Unmated, mature worms ( Fig. 11F View Fig ) have tubular atria with a thick epithelium, whereas sac-like atria with thin walls lacking a glandular epithelium or an obvious muscle layer were observed in worms at a more advanced stage of maturity ( Fig. 11E, H View Fig ), including post-reproductive specimens with partially-resorbed reproductive organs.
Habitat
All sites except the spring on Peavine Ridge were alluviated streams with gravel to cobble substrate. All appear to have permanent flow, except for Guadalupe and Alamitos Creeks, where surface flow may disappear during summer months. Worms were typically collected by digging at least 20 cm deep in patches of finer gravel.
The Clearwater drainage sites, on the Olympic Peninsula, northwestern Washington, are larger streams in a watershed dominated by commercial forest, but with riparian buffers. These streams support populations of several salmonid species ( Harrington 2005). Limited available water quality data for the Clearwater River near Clearwater ( NWIS 2016b) indicate low specific conductance (measured in 1972– 1974: 13–94 μS cm-1). Guadalupe, Alamitos and Euchre Creeks are small, coastal drainages ranging from southern Oregon to central California. The Peavine Ridge collection was from an isolated springfed, seasonally inundated pool with fine sediment, and the species was found on only one of several visits. Values for specific conductance in both the Peavine Ridge spring and in Euchre Creek (measured in April 2014: 95 and 63 μS cm-1, respectively) were low. However, values tend to be much higher (at summer base flow, 340–490 μS cm-1) in Guadalupe and Alamitos Creeks (unpublished field data, J.L. Carter, US Geological Survey, 3 sampling dates in May, June, September 1997–1998).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Guestphalinus elephantinus Fend & Rodriguez
| Fend, Steven V., Rodriguez, Pilar, Achurra, Ainara & Erséus, Christer 2017 |
Guestphalinus
| Zhou H. & Fend S. V. & Gustafson D. L. & De Wit P. & Erseus C. 2010: 381 |