Kincaidiana hexatheca Altman, 1936
|
publication ID |
https://doi.org/ 10.5852/ejt.2017.361 |
|
publication LSID |
lsid:zoobank.org:pub:F61CB5C7-B22E-4FAB-997A-BF99C7828C77 |
|
DOI |
https://doi.org/10.5281/zenodo.3851846 |
|
persistent identifier |
https://treatment.plazi.org/id/03BC8649-FF99-FF9E-FDED-FCF2DA94B7C1 |
|
treatment provided by |
Carolina |
|
scientific name |
Kincaidiana hexatheca Altman, 1936 |
| status |
|
Kincaidiana hexatheca Altman, 1936 View in CoL
Figs 1–3 View Fig View Fig View Fig
Kincaidiana hexatheca Altman, 1936: 64–68 View in CoL , figs 53–59, 66.
Kincaidiana hexatheca View in CoL – Brinkhurst & Cook 1966: 10, figs 2A, 5B, D, I. — Cook 1971: 237, figs 5.4 F–I, 5.5 D. — Fend 2009: 3–6 View Cited Treatment , figs 1–2.
Material examined
Lectotype
UNITED STATES OF AMERICA: a sagittally sectioned worm on 4 slides, Series II, from the Altman collection, Washington, Pacific County, Loomis Lake , 11 Sep. 1929 ( UWBM).
Other type material (Altman collection, UWBM)
UNITED STATES OF AMERICA: 1 dissected, same locality as lectotype, 14 Nov. 1931; 1 dissected, same locality as lectotype, 26 May 1932; 1 dissected, undetermined location (possibly Loomis Lake), 6 Apr. 1931; 1 dissected, undetermined location (possibly Loomis Lake), 23 Apr. 1931; 1 dissected, no date (#199); additional syntypes were examined by Fend (2009, figs 2D–E, G–H).
New collections (mature specimens, unless otherwise noted)
UNITED STATES OF AMERICA: WASHINGTON: 2 sagittally sectioned, 2 dissected, several in alcohol, Jefferson County, small seep along Hoh River Road, 29 Apr. 1999, S. Fend leg.; 1 dissected, 2 in alcohol, same locality as preceding, 26 Apr. 2004, S. Fend leg.; 3 dissected, Hoh River drainage, roadside ditch, on Clearwater Road, 3 Jun. 2003, S. Fend leg.; 1 dissected, Pacific County, spring on Naselle River, 30 Apr. 1999, S. Fend leg.; 1 dissected, Clallam County, small pool along Bogachiel River on Undi Road, 26 Apr. 2004, S. Fend leg.; 1 mature, 3 post-mature, dissected, Clark County, Salmon Creek watershed, 10 Sep. 2001, R. Wisseman leg.; 2 partially-mature, dissected, Skamania County, Dog Creek near mouth, 26 Apr. 2014, P. Rodriguez and S. Fend leg. – OREGON: 2 dissected, 1 whole mount, Multnomah County, Oneonta Creek near mouth, downstream of Oneonta Gorge parking area, Columbia Gorge, 4 Jun. 2003, S. Fend leg.; 1 dissected (partially-mature), 1 whole mount, Tillamook County, small spring on Nestucca River 0.4 miles above Blaine, on Bible Creek Road, near Tillamook, 4 Jun. 2003, S. Fend leg.; 3 dissected, several in alcohol, Yamhill County, seep on east side of McGuire Reservoir near McMinnville, 4 Jun. 2003, S. Fend leg.; 7 dissected, several in alcohol, Lane County, muddy seep at Big Creek, FR5700, 11 May 2001, S. Fend leg.; 1 dissected, 1 in alcohol, same locality as preceding, 28 Apr. 2014, S. Fend leg.; 1 dissected, 4 immature, slide mounts, Lane County, small spring at mouth of Tenmile Creek, 1 May 1999, S. Fend leg.; 1 whole mount (immature), Lane County, marsh at base of Mt Pisgah, near Eugene, 29 Jan. 2000, S. Fend leg.; 6 dissected, Lane County, outflow from Leaburg fish hatchery (McKenzie River), 19 May 2013, S. Fend leg.; 1 dissected, 2 in alcohol, Whittaker Creek at Siuslaw River, 12 Aug. 2016, S. Fend leg.; 3 immature whole mounts (1 is DNA voucher CE 861), Douglas County, Cow Creek, tributary to Umpqua River, 28 Apr. 2004, S. Fend leg.; 6 dissected, 2 in alcohol, Douglas County, spring above Mule Creek, 7 Jun. 2003, S. Fend leg.; 1 transverse section, 4 dissected, 5 whole mounts, Josephine County, Darlingtonia bog near O’Brien, off Wimer-Lone Mt Road, 26 Oct. 1999, S. Fend leg.; 1 whole mount, Josephine County, Illinois River near Sixmile Creek, 25 Oct. 1999, S. Fend leg.; 1 whole mount, same locality as preceding, 7 Jun. 2003, S. Fend leg.; 1 dissected, 1 whole mount, same locality as preceding, 21 May 13, S. Fend leg.; 3 dissected, 1 whole mount, 3 immature dissected, Curry County, Rogue River at Quosatana Campground ( NFS) ca 19 miles above Gold Beach, 8 Jun. 2003, S. Fend leg. – CALIFORNIA: 9 dissected (plus 3 immature dissected), Mendocino County, Inglenook Fen at McKerricher State Park, slow creek, roots of Apiaceae , 1 Jul. 2005, S. Fend leg.; 1 dissected DNA voucher, same locality as preceding, 7 Jul. 2006, S. Fend leg. ( CE 2289).
Molecular data
COI, 16S, and 28S sequences are from two specimens, collected at Inglenook Fen, California and Cow Creek, Oregon (details in Table 1 View Table 1 ).
Description of new material
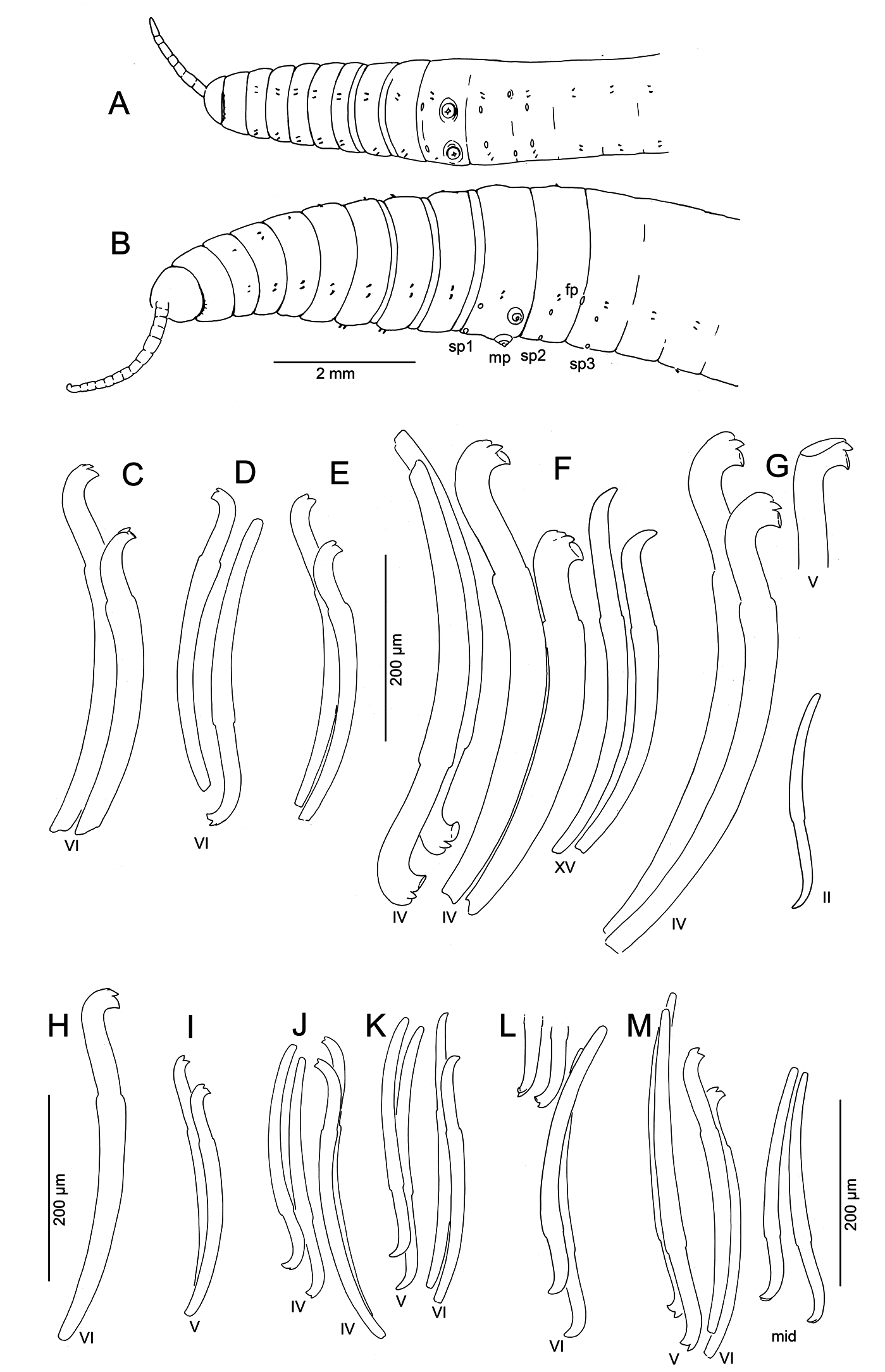
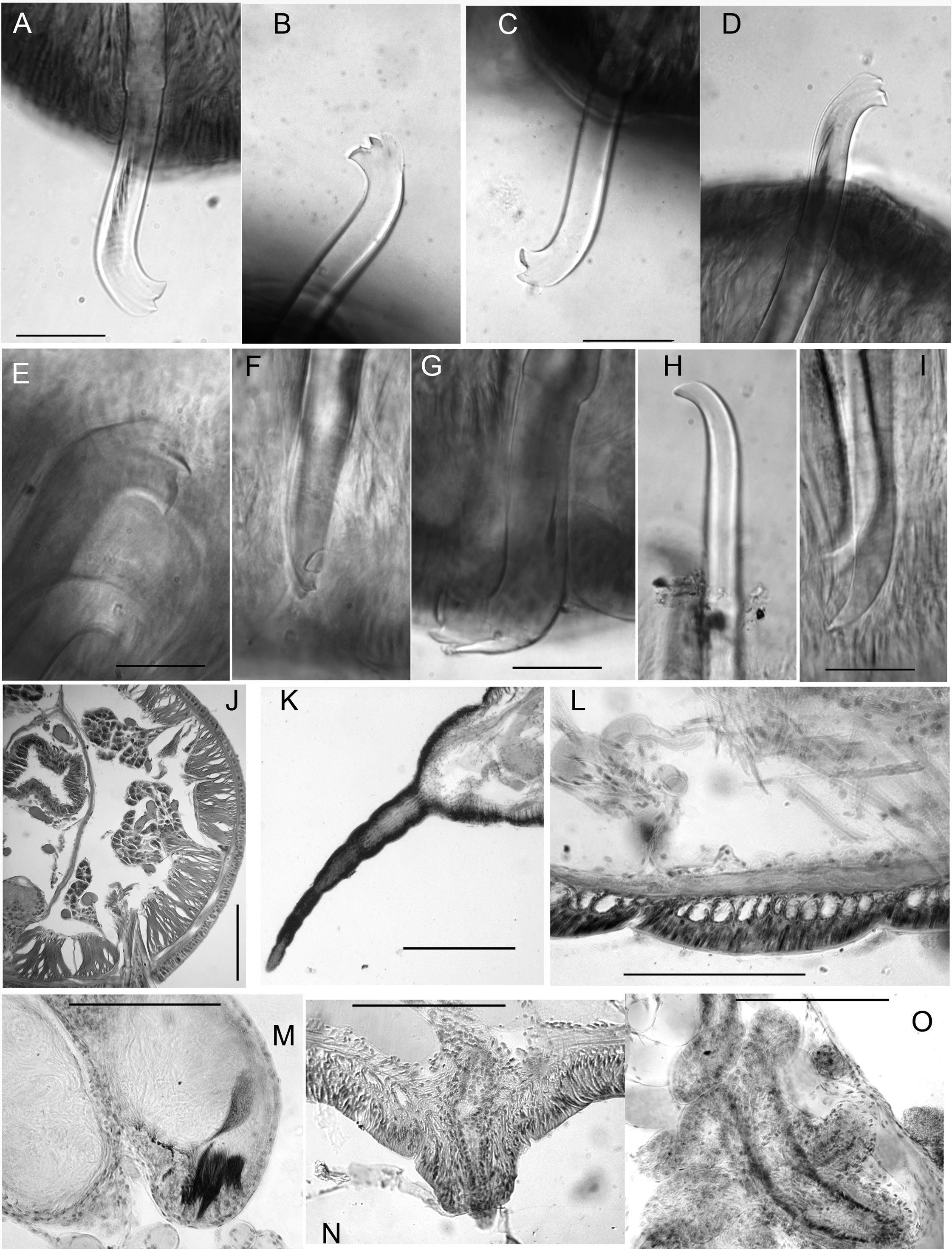
Size variable ( Table 2 View Table 2 ); specimens from swampy habitats usually larger than those from gravel-bed streams; largest specimens (length> 100 mm, diameter> 2 mm, segments> 200, Fig. 1B View Fig ) from muddy seeps in Oregon. No obvious latitudinal difference in size (see length, width and segment numbers in Table 2 View Table 2 ). Largest specimens considerably larger than material collected by Altman (1936) (diameter 0.75–1.25 mm) from two sites in Washington and one in Oregon. Proboscis elongate, appearing ringed externally, but lacking internal septa ( Figs 1 View Fig A–B, 2K).
Chaetae in anterior segments (II to VI, VII or VIII) almost always modified, with distal ends oriented anteriad. Modified anterior chaetae may appear simply bifid in lateral view, with short upper tooth ( Figs 1C View Fig , 2C View Fig ), but structure usually more complex: lower tooth broad and flattened or concave, and thin dorsal keel may extend beyond upper tooth ( Figs 1 View Fig F–G, 2A–B, E–G). Keel visible as translucent outer edge in lateral view, or narrow point in frontal view ( Fig. 2F View Fig ); usually most prominent on replacement chaetae, but absent on many chaetae, possibly due to wear; keel often broken or folded over ( Figs 1G View Fig , 2G View Fig ) in mounted specimens, with chaeta appearing trifid. This structure occurs throughout the geographic range of the species, but not always visible in specimens from some populations. Chaetae in atrial and postatrial segments usually simple-pointed, moderately sigmoid and oriented posteriad; tips slightly keeled in posterior segments on some specimens ( Fig. 1M View Fig ).
Chaetae in segment II always smaller than those in next several segments; ventrals in II usually simplepointed. Chaetae from III to about VII or VIII usually thicker than (but similar in length, see Table 2 View Table 2 ) to those in following segments, with more distal nodulus; distinctly longer in some individuals, and up to 50% longer in Big Creek (west-central Oregon) worms. Within a bundle, inner chaeta typically longer than outer, with more proximal nodulus ( Fig. 1C, F View Fig ). Greatest modification in size, position of nodulus, and development of teeth in anterior chaetae in specimens from Big Creek ( Figs 1 View Fig F–G, 2E–G).
Pharynx with high columnar cells from II to IV, with dorsal and lateral epithelium higher than ventral. Intestine begins in 6/7. Pharyngeal glands in V or VI to VII or VIII, on each side produced into 3 irregular, anteriorly directed lobes ( Fig. 2J View Fig ) joining at the base (posterior septum). Contrary to the description by Altman (1936), they were never observed in II or III, and are about equally distributed dorsally and ventrally. Nephridia begin on 11/12, with narrow, dorsally-directed postseptale, as described by Fend (2009). Circular muscle layer of body wall forms distinct bands in anterior segments ( Fig. 2L View Fig ).
Lateral trunks of dorsal blood vessel join to form ventral vessel in III or IV, anterior to location in Altman’s description (V). Unbranched, but highly convoluted commissural vessels in anterior segments to about XX. One or two pairs of lateral vessels in segments posterior to about XX; morphology of these vessels variable in middle segments; most commonly, the anterior is larger, branched and covered in chloragogen cells; alternatively, the posterior pair may be branched, and the anterior pair simple, as stated by Altman (1936). Posterior segments may have two pairs of branched vessels.
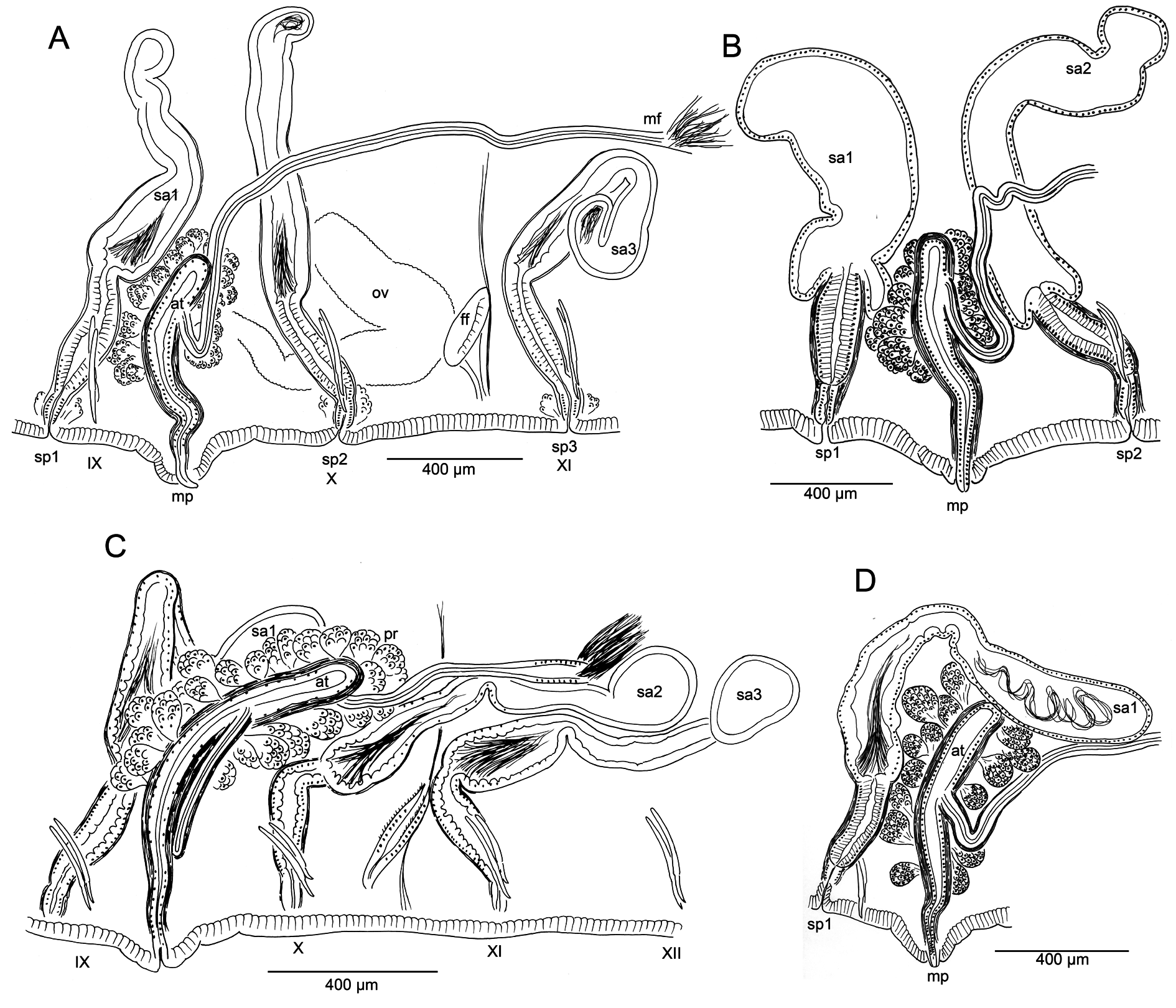
Male pores always paired, median and posterior to ventral chaetae on IX ( Fig. 1 View Fig A–B); conical porophores developed ( Fig. 2N View Fig ) or not ( Fig. 3 View Fig B–C). Spermathecae paired in IX, X, and XI; spermathecal pores slightly displaced towards ventral midline; pores in IX slightly anterior to chaetae, those in X–XI level with chaetae ( Fig. 1 View Fig A–B).
Atria generally more elongate-tubular than in the illustration by Altman (1936: fig. 59) ( Fig. 3 View Fig ). Atrial ampulla and ectal duct weakly differentiated, ampulla distinguished only by slightly greater diameter and presence of prostates ( Fig. 2O View Fig ). Atrium length, including length relative to body width, shows overlap among regions ( Table 2 View Table 2 ). Atria usually entirely in IX, but extend into X in four specimens from sites throughout the species distribution ( Fig. 3C View Fig ). Male funnels may be displaced back within sperm sacs as far as XI ( Fig. 3A, C View Fig ) or even XII.
Spermathecal duct about 300–600 µm long, tubular, histologically differentiated from ampulla, having thick, irregular epithelium of columnar cells and more well-developed muscular layer. Duct may be sharply constricted at ectal end, as it joins a narrow epidermal infolding. Spermathecal ampulla 800– 1600 µm long; sharply expanded in basal part in some specimens ( Fig. 3B View Fig ), as in the original description ( Altman 1936: fig. 57), but more typically elongate-tubular ( Fig. 3A View Fig , C–D). Sperm usually lined up along epithelium near ectal end of ampulla ( Figs 2M View Fig , 3D View Fig ), absent in the duct.
Remarks
The type series was not clearly designated by Altman (1936), and material used in the original description appears to have been collected from 3 sites in southwestern Washington and northwestern Oregon. The sagittally sectioned specimen here designated as the lectotype ( Fig. 2A View Fig , K–O) shows most of the diagnostic characters: annulated proboscis, bifid/keeled anterior chaetae, spermathecae paired in atrial and 2 postatrial segments, conical male porophores, short-tubular atria with prosopore male ducts paired in IX. Other worms from the type series (mostly from undetermined localities) also show these characters (see fig. 2D–E, G in Fend 2009).
In addition to examining 18 apparent syntypes, Fend (2009) verified diagnostic characters in specimens from additional sites in Washington, Oregon, and California, but did not discuss regional or population differences. Here we examine variation in morphological characters in specimens from throughout the known geographic distribution of the species and molecular differences between specimens from two sites in the southern part of the range, near the type locality of Kincaidiana smithi sp. nov. (see below).
The bifid, anteriorly-directed chaetae in preclitellar segments are one of the most distinctive characters for K. hexatheca . Despite some population differences in size of these chaetae, the general pattern was consistent in most populations. However, the occurrence of worms from a few sites in southwestern Oregon, having typical K. hexatheca reproductive organs, but with simple-pointed or only slightly bifid anterior chaetae, cautions against reliance on this character alone for identifying immature specimens. All chaetae (including replacements) were simple-pointed in a partially-mature specimen from Cow Creek (Umpqua River drainage), and immature worms from the same site were similar; chaetae in the first few anterior segments were not enlarged, although they were anteriorly-directed. This condition was variable in mature and immature specimens from two sites in the Rogue River drainage: a spring near Mule Creek ( Fig. 1 View Fig J–K) and the Illinois River ( Fig. 1L View Fig ); in one such specimen the replacement chaetae are bifid ( Fig. 1L View Fig ), but in other individuals all chaetae (including replacements) were simplepointed. This suggests that simple-pointed chaetae do not simply reflect wear in worms from gravel-bed streams.
There was no obvious latitudinal difference in size and exent of modification of anterior chaetae ( Table 2 View Table 2 ). The maximum chaeta length in anterior segments of several populations was considerably larger than the 0.218 mm reported by Altman (1936).
Morphology of reproductive organs varied within populations ( Fig. 3 View Fig ), possibly masking regional differences. Our observations differ from prior descriptions in minor details. Cook (1971) stated that spermathecal pores are behind the ventral chaetae in IX, but Altman (1936) placed them “between, and just ventral” (median?) to the ventral chaetae. All of the new material has spermathecal pores distinctly displaced towards the ventral midline, and the first pair is clearly anterior to the ventral chaetae ( Fig. 1 View Fig A–B). Atrium length varied by about a factor of 2 within each region, although this was less when normalized by body diameter ( Table 2 View Table 2 ). The limited data suggest that atria of specimens in the California population were larger relative to body size. Spermatheca size varied similarly due to the elongate, irregular ampullae; however, the duct length was less variable.
The species appears to be endemic to the Pacific northwestern USA and British Columbia ( Kathman & Brinkhurst 1998); reported records from other regions cannot be verified, as material was mostly unavailable for study. Some confusion may be based on ambiguous somatic characters regarded as distinctive in published keys (e.g., Kathman & Brinkhurst 1998). In particular, wrinkling due to fixation of other proboscis-bearing species may be interpreted as a “pseudo-segmented” proboscis, and minutely bifid chaetae (possibly a result of wear) on some specimens of Rhynchelmis may also cause confusion. For example, Spencer & Denton (2003) tentatively attributed immature specimens from Utah to K. hexatheca , but recent examination by one of the authors (S. Fend) of some of this material deposited in the Bean Life Science Museum (Brigham Young University, Provo, Utah) suggested that they were more likely to be a species of Rhynchelmis . Therefore, morphology-based identification of immature specimens of K. hexatheca should ideally be based not only on the proboscis and presence/ absence of modified anterior chaetae, but also on other morphological characters. For example, two pairs of lateral blood vessels in segments X–XX of the Nearctic Rhynchelmis species (if present) are short, usually branched, and do not join the ventral vessel ( Fend & Brinkhurst 2000).
Habitat
Kincaidiana hexatheca has been collected in many coldwater habitats, ranging from cobble riffles in large streams to small, muddy seeps, typically associated with aquatic plants (e.g., skunk cabbage, Lysichiton americanus Hultén & St. John and water parsley, Oenanthe sarmentosa Presl ).
| UWBM |
University of Washington, Burke Museum |
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Kincaidiana hexatheca Altman, 1936
| Fend, Steven V., Rodriguez, Pilar, Achurra, Ainara & Erséus, Christer 2017 |
Kincaidiana hexatheca
| Fend S. V. 2009: 3 |
| Cook D. G. 1971: 237 |
| Brinkhurst R. O. & Cook D. G. 1966: 10 |
Kincaidiana hexatheca
| Altman L. C. 1936: 68 |