Raphidrilus harperi, Magalhães, Wagner F., Bailey, Julie H., Brock, - & Davenport, Jennifer S., 2011
|
publication ID |
https://doi.org/ 10.5281/zenodo.277061 |
|
DOI |
https://doi.org/10.5281/zenodo.5631414 |
|
persistent identifier |
https://treatment.plazi.org/id/03C587D3-2247-FFFE-E4D3-2E7C766CFB68 |
|
treatment provided by |
Plazi |
|
scientific name |
Raphidrilus harperi |
| status |
sp. nov. |
Raphidrilus harperi sp. nov.
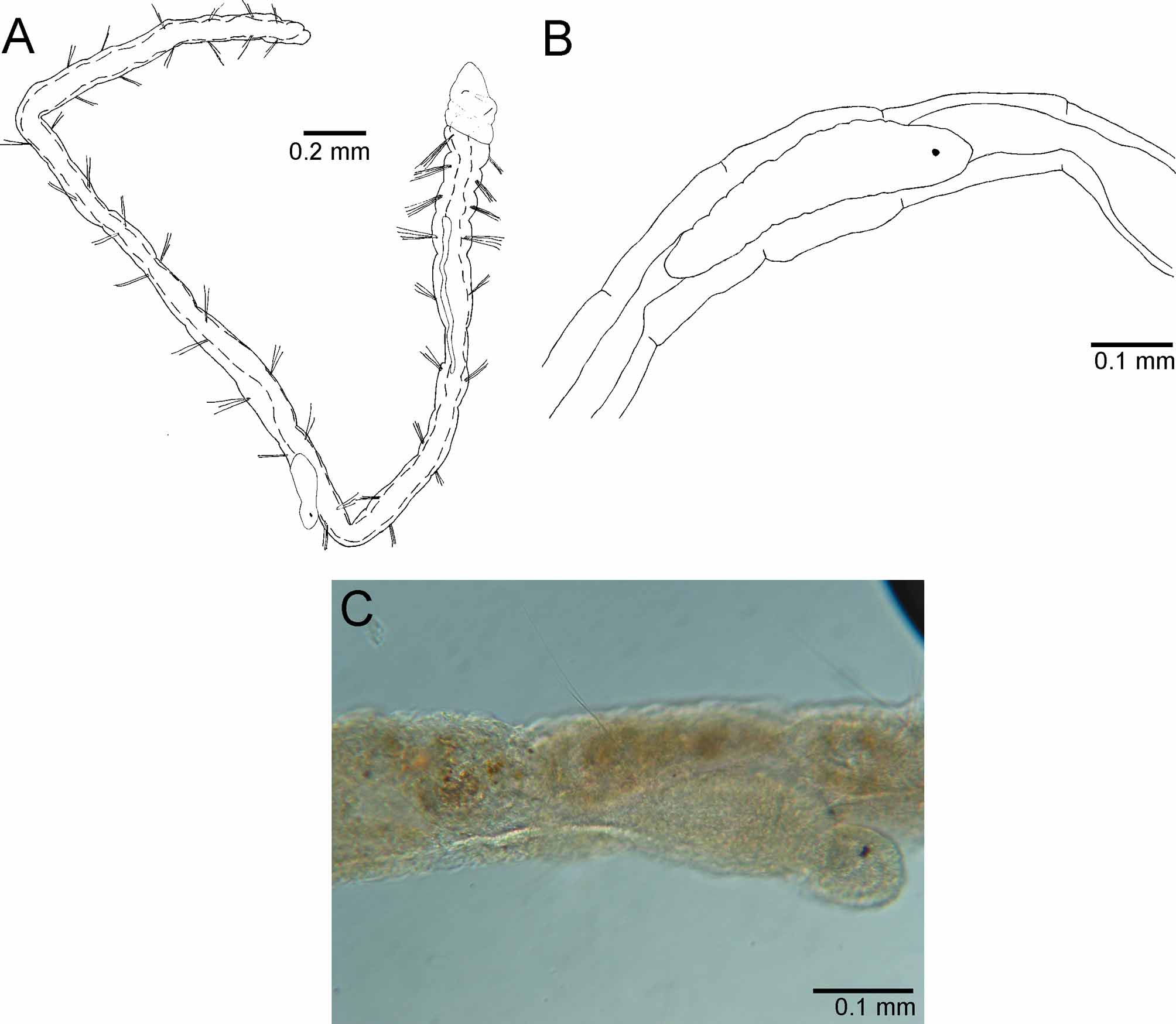
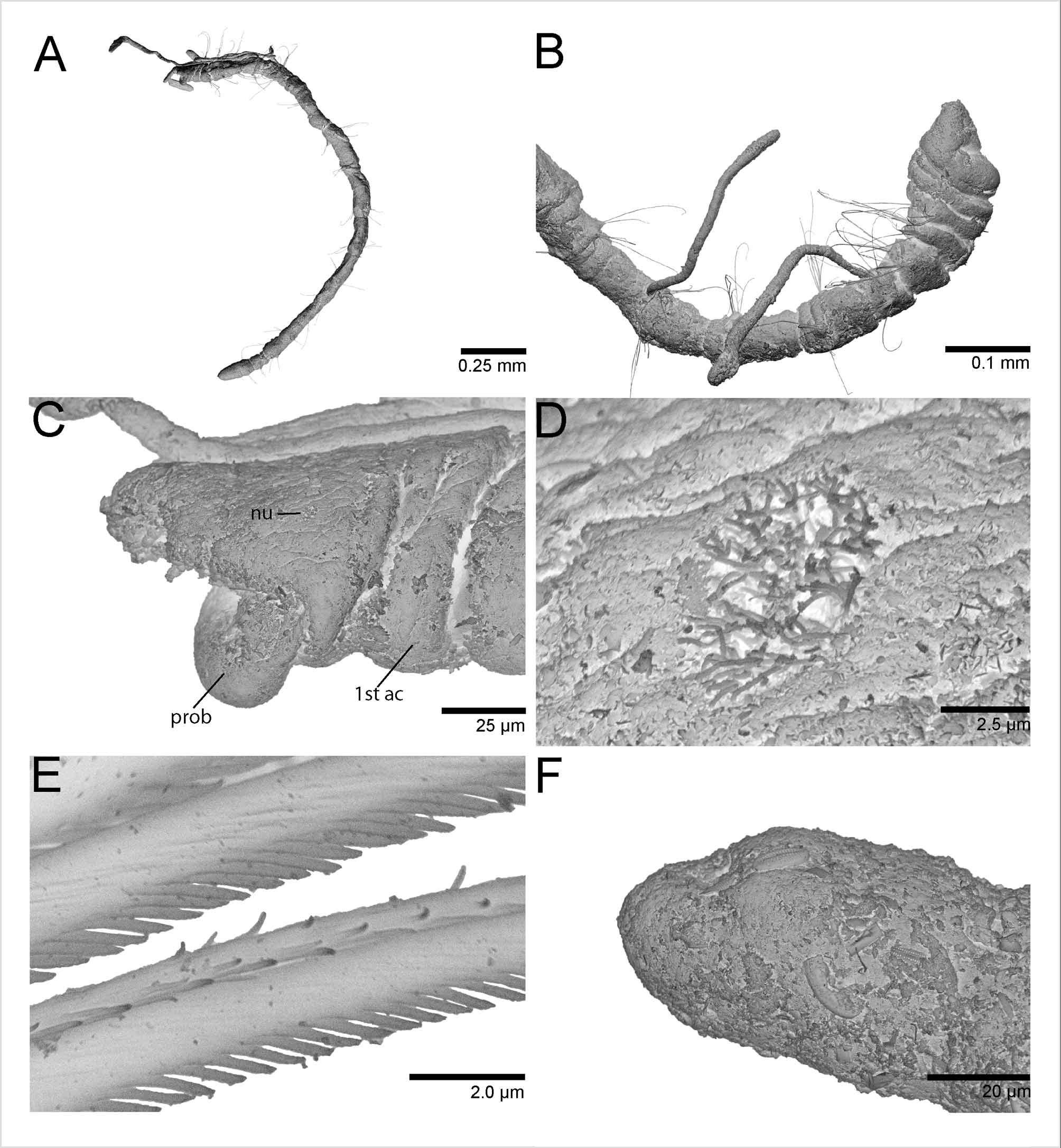
Figures 2 View FIGURE 2 (A–C) and 3 (A–F)
Material examined. Holotype: GIWW at Venice, Florida, USA, 27º06’01.3” N, 82º26’08.5” W, Station 5NB, coll. D. Seagle, August 28, 2009 ( USNM 1150467). Paratypes: same location, date and collector as holotype, Station 5NB (9, USNM 1150469); Stations 5NB and 5NC (4, FSBC I 072250); Station 5NB (4, MAGNT W23467– W23470); Station 5NB (1 mounted on stub, BMNH 2011.7); Station 5NB (2 mounted on stub, USNM 1150468). Non– type material: same location, date and collector as type –series, Station 5NB (3); Station 5NC (1).
Description. Complete specimens ranged from 3.1–5.8 mm in length, 0.1–0.25 mm in width and possessed between 17–27 chaetigers. Body thin, cylindrical, and elongated. First four chaetigers (thorax) and last few wider than long; abdominal chaetigers vary from 1.5–4 times longer than wide ( Fig. 2 View FIGURE 2 A). Color in alcohol light yellow to brown.
Prostomium short, broadly rounded with pair of postero–lateral nuchal organs ( Fig. 3 View FIGURE 3 C). Nuchal organs oval ciliary patches (~7 µm) with long cilia, situated in a shallow depression ( Fig. 3 View FIGURE 3 D). Distinction between prostomium and peristomium inconspicuous laterally; peristomium single achaetous segment, appearing pear–shaped together with the prostomium; a single biannulated achaetous segment following the peristomium ( Fig. 3 View FIGURE 3 B). Heart body usually begins in chaetiger 4, but sometimes projects anteriorly into the posterior region of chaetiger 3; usually extends to chaetiger 7, but occasionally continues to chaetiger 9 in the material examined ( Fig. 2 View FIGURE 2 A). Branchial filaments postero–dorsal to notochaetae, easily broken and occurring in pairs or singly from chaetigers 3 through 11 ( Fig. 3 View FIGURE 3 A, B).
Anterior chaetigers with 3–6 serrated capillaries in both noto– and neurochaetal fascicles; posterior chaetigers with 1–4 capillaries per fascicle. Serrations of some capillaries evident using phase contrast microscopy with oil immersion at 1000x magnification; SEM revealed fibrils along capillary edge with distance between the insertion point of two capillary fibrils approximately the same as half the width of a single fibril ( Fig. 3 View FIGURE 3 E).
Pygidium elongated segment with dorsal anal aperture; fields of cilia not observed ( Fig. 3 View FIGURE 3 F).
Etymology. This species is named in honor of the third author’s graduate advisor, Dr. Donald E. Harper, Jr., Professor Emeritus of Texas A&M University at Galveston. Dr. Harper graciously introduced me to the world of polychaetes and has provided valuable guidance and encouragement over the years.
Biology. Raphidrilus harperi sp. nov., was collected just north of a reverse osmosis plant outfall. Water quality data is typical for a shallow, estuarine waterbody during late summer in southwest Florida ( Table 1 View TABLE 1 ). All specimens were sexually immature and regenerating specimens were not observed. Segmented worms were observed, however, in the coelom of several specimens and were oriented in both directions along the anterior–posterior axis of the host ( Fig. 2 View FIGURE 2 B, C). Segmented worms were dissected out of the host and neither chaetae nor branchiae were observed. These segmented worms may be an intracoelomic parasite or gestational larvae resulting from sexual reproduction. Petersen and George (1991) indicated protandric hermaphroditism with internal gestation for R. nemasoma . Additionally, Sokolow (1911a, fig. 77) illustrated juveniles emerging from the coelom of a parent, which appear quite similar to the segmented worms observed in R. harperi sp. nov. ( Fig. 2 View FIGURE 2 A–C). Future in vivo investigations would help resolve the unknown reproductive processes of R. harperi sp. nov.
Distribution. Raphidrilus harperi sp. nov., is known only from the type locality, the GIWW in Venice, Florida. The distribution of this species is suspected to extend further south into the Florida Keys (T. H. Perkins, pers. comm.) if it is the same species that Petersen and George (1991) referred to in their study. Unfortunately, those specimens have been lost (T. H. Perkins, pers. comm.), and could not be observed for comparison. Based upon correspondence and associated drawings between T. H. Perkins and M. E. Petersen specimens from the Florida Keys superficially appear to be R. harperi sp. nov. The general body shape, number of chaetae per fascicle and the description of chaetae all match R. harperi sp. nov. The only difference is that no branchiae or scars of branchiae were observed in the specimens from the Florida Keys, whereas almost all specimens from Venice have at least a stub, a single branchial filament or multiple branchial filaments. Additional specimens from the FSBC I collections labeled as “ Raphidrilus sp.” were examined for comparison (FSBC I 45229 View Materials and FSBC I 45230 View Materials ). These specimens were collected from Broward County along the east coast of Florida and are not R. harperi sp. nov. They possess pectinate falcigers in the middle and posterior chaetigers and are most likely an undescribed species of Raricirrus . Future collection efforts along the Gulf coast of Florida would help determine the geographical distribution of R. harperi sp. nov.
Remarks. Table 2 View TABLE 2 summarizes the morphological characters useful to separate species in the genus Raphidrilus . Raphidrilus harperi sp. nov., differs from R. hawaiiensis sp. nov., and R. nemasoma by the presence of 3–6 capillary chaetae per fascicle in anterior chaetigers, while in both R. hawaiiensis sp. nov., and R. nemasoma the number of capillaries per fascicle is never greater than 4. The elongated mid–body and posterior segments in R. harperi sp. nov., is also very distinct and lack sub–annulations present in R. hawaiiensis sp. nov., and R. nemasoma . The heart body in R. harperi sp. nov., extends posteriorly to chaetigers 7–9, while in R. hawaiiensis sp. nov., the heart body projects anteriorly to the middle of chaetiger 3 and in R. nemasoma the heart body is restricted to the extension of chaetiger 4 ( Monticelli 1910b).
The species of Raphidrilus referred to by Qian and Chia (1989) as Raphidrilus nemasoma , and later considered to be undescribed by Petersen and George (1991), is distinct from R. harperi sp. nov., R. hawaiiensis sp. nov., and R. nemasoma . Even though adult worms of Raphidrilus sensu Qian and Chia (1989) were not described by these authors, the many scattered short sensory cilia in addition to the nuchal organs present on the prostomium and peristomium, the sensory tufts postero–dorsal to notochaetae, the short serrate neurochaetae (reported as being genital spines), and the terminal anus of juveniles worms (8–11 chaetigers) are unique characteristics not observed in the species from Florida, Hawaii, or the Mediterranean Sea. Adult specimens from the same locality sampled by Qian and Chia (1989) need to be examined to confirm the status of a new species.
* based on juveniles with 8–11 chaetigers.
continued.
TABLE 1. Bottom water quality parameters for the sampling station where Raphidrilus harperi sp. nov., and R. nemasoma were collected.
| Species | Depth (m) Water Temper- ature (ºC) | Salinity (ppt) | Dissolved Oxy- gen (mg/L) | Specific Conduc- tivity (μmho/cm) | pH | Chlorides (g/ Kg) |
|---|---|---|---|---|---|---|
| R. harperi sp. nov. | 2.7 30.0 | 26.1 | 3.58 | 40,600 | 7.3 | 14.4 |
| R. nemasoma | 6.4 14.0 | 37.725 | – | – | – | – |
TABLE 2. Taxonomic characters of four Raphidrilus species including one undescribed species from the western Pacific.
| Species | Prostomium | Peristomium | Achaetous segment | Position and extent of heart body |
|---|---|---|---|---|
| R. harperi sp. nov. | Rounded; pair of oval nuchal organs; pear–shaped with peris- tomium | Single annulus; not dor- sally delimited from pros- tomium | One dorsally biannu- lated | Chaetiger 4; posteriorly directed to chaetiger 7 |
| R. hawaiiensis sp. nov. | Rounded; pair of oval nuchal organs; pear–shaped with peris- tomium | Single annulus; not dor- sally delimited from pros- tomium | 1–2 dorsally biannu- lated | Chaetiger 4; anteriorly directed to middle of chae- tiger 3 |
| R. nemasoma Monticelli, 1910 | Broadly rounded; a pair of nuchal organs; thimble–shaped with peristomium | Single annulus; not dor- sally delimited from pros- tomium | One dorsally biannu- lated | Chaetiger 4 only |
| R. sp. sensu Qian & Chia, 1989* | Broadly rounded; a pair of nuchal organs and scattered sen- sorial tufts; thimble–shaped with peristomium | Single annulus? | One dorsally biannu- lated? | ? |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.