Drosophila (Sophophora) schugi, McEvey & Schiffer, 2015
|
publication ID |
https://doi.org/ 10.3853/j.2201-4349.67.2015.1651 |
|
publication LSID |
lsid:zoobank.org:pub:EAD7EB42-7702-4CD1-B2FC-5B6845E3D9BC |
|
persistent identifier |
https://treatment.plazi.org/id/219CD39F-032E-4FBE-80C3-21BC38542AFD |
|
taxon LSID |
lsid:zoobank.org:act:219CD39F-032E-4FBE-80C3-21BC38542AFD |
|
treatment provided by |
Felipe |
|
scientific name |
Drosophila (Sophophora) schugi |
| status |
sp. nov. |
Drosophila (Sophophora) schugi sp.nov.
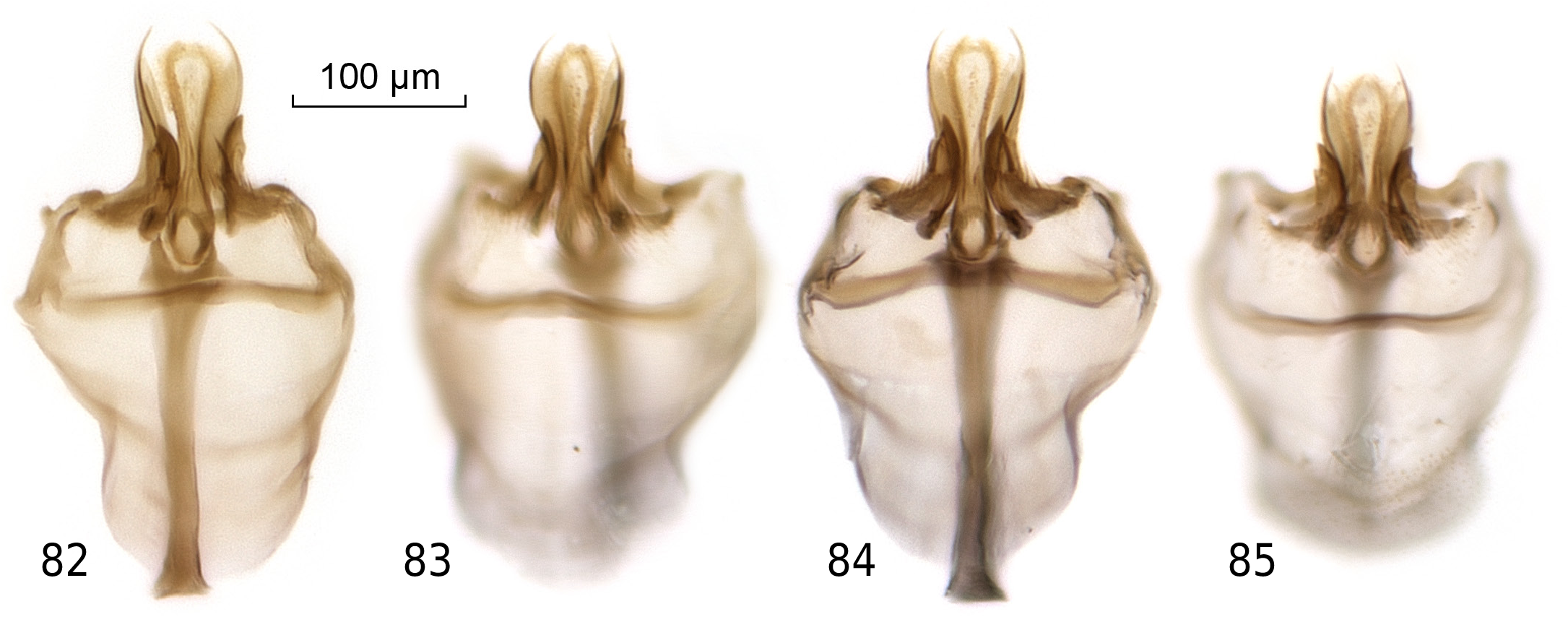
Figs. 13, 49, 53, 66–71, 82–88
Types. Holotype ♂, AMS K282922 , McEvey 21326, “ WESTERN SAMOA | Malololelei, Upolu | 14–17 June 2003 | coll. S.F. McEvey [with M. Schug, Shelly Gray- Smith, M. Marshall]” . Paratypes (12♂♂), 8 pinned AMS K282919–921 , K282923–925 , K282929–930 ; 4 in alcohol AMS K356976–979 (terminalia dissected); all same data as holotype. No cultures established. Terminalia of K282923 dissected and mounted on slide. All in the Australian Museum .
Distinguishing features. Sex combs of male forelegs very strongly developed on first three tarsal segments, tarsomere III in two rows of 2–4 teeth each; anterior parameres large, scimitar-shaped or with ragged lateral edge; caudal margin of novasternum with no medial convexity.
Description (♂)
Body length. 2.2 mm ♂.
Head ( Fig. 72–74 View Figures 72–77 ). Arista with 4 rays above and 2–3 below, plus terminal fork. Orbital setae in ratio 4:2:5. Carina ( Fig. 72, 74 View Figures 72–77 ) prominent, dorsally with narrow ridge. Frons narrow anteriorly, broad posteriorly. Greatest width of gena less than 0.1 greatest diameter of eye. (Measurements of holotype ♂)—BL(McE) = 2.19 mm, BL(Z&T) = 1.58 mm, hw/fw(ov) = 1.93, hw/fw(iv) = 2.28, hw/fw(vt) = 1.91, hw/fw(a.oc) = 2.07, hw/fw(a. r.orb ) = 2.34, hw/fw(ptl) = 2.62, fw(ov)/ fl = 1.33, fw(iv)/fl = 1.13, fw(vt)/fl = 1.35, fw(a.oc)/fl = 1.25, fw(a. r.orb )/fl = 1.10, prorb = 0.85, rcorb = 0.47, proc. orb/a. r.orb = 1.83, oc/proc.orb = 1.13, pv/oc = 0.75, p. r.orb /iv = 0.74, orbito-index = 0.96, vt-index = 1.04, oc-gap/pv-gap = 0.44, o/j = 29.0, ch/o = 0.05, o/ow = 1.29, svb/vb = 0.89, flw = 1.45, avd = 0.99, adf = 1.66, arista free ends = 9.
Thorax. Acrostichal hairs in 8 rows in front of dorsocentral bristles. Ratio anterior/posterior dorsocentrals 0.6. Preapical bristles on all tibiae; apicals on first and second tibiae. Sex comb of male foreleg ( Figs. 50–55 View Figures 38–53 View Figures 54–71 , Table 1) in transverse rows (from above down) 6–8 rows on tarsomere I of 0–1, 0–2, 2–4, 3–4, 3–6, 6–7, 6–8, and 6–7 teeth; tarsomere II with 5–6 rows of 0–2, 1–4, 4–6, 5–6, 5–7 and 4–6; and
Kota Kinabalu Townsville, Qld
D. parapallidosa D. pandora sp.nov.
Wau, PNG Bulolo, PNG D. pandora sp.nov. “ D. pallidosa -like-WAU ”
tarsomere III in 2 rows of 2–4 and 2–3 rows. Other thoracic measurements (holotype ♂)—pre-sc/pdc = 0.25, bsc/asc = 0.82, sterno-index = 0.61, m/a.kepst = 0.61, p.kepst/pdc = 1.12, pdc/asc = 0.81, asc–bsc/asc–asc = 1.19, a–pdc/dc-gap = 0.38, adc/pdc = 0.62, fw(a.oc)/dc-gap = 1.21.
Wing. Hyaline, wing length c. 1.9 mm. L(Ax) = 1.88 mm, WL = 1.71 mm, L 1 = 1.66 mm, L(Ax)/WW = 2.37, WL/WW = 2.16, L 1 /WW = 2.10, C-index = 1.58, 4v-index = 2.60, 4c-index = 1.66, 5x-index = 2.48, M-index = 0.94, ac-index = 3.50, C3 fringe = 0.52.
Abdomen. Tergites dark especially posteriorly, apical tergites darker, tergite VI usually dark brown.
Male terminalia. Epandrium (periphallic organs) ( Fig. 49 View Figures 38–53 ) narrow dorsally and ventrally; toe elongate, with about 12 setae apically. Primary clasper (surstylus) and secondary clasper present. Surstylus large, with two sets of teeth— medial row of 4 strong pointed upper teeth and a cluster of about 8 lower pointed teeth (one greatly elongated, curved towards decasternum); and a lateral row of thicker, blunt, black, teeth in 2 sets: an upper of 2 (widely spaced) and a lower of 3–5 tightly spaced teeth. Secondary clasper small, with a very large curved black medial tooth, and about 3 small lateral setae.
Hypandrium (phallic organs). Aedeagus brown, nonbifid, narrowed in middle, and strongly hirsute in apical third (Figs 13, 68–71); aedeagal apodeme as long as the ventral phragma and considerably expanded in lateral view. Anterior parameres large, scimitar-shaped (Fig. 13) or with ragged lateral edge ( Figs. 82–85 View Figures 82–85 ), articulated to aedeagus, and laterally with no minute sensilla. Posterior parameres strongly tapering apically, long, extending past tip of aedeagus. Caudal margin of novasternum ill-defined, slightly hirsute, with no medial convexity, and with a pair of submedian spines not widely separated. Hypandrium with transverse band (example marked tb in Fig. 4) nearly as wide as ventral phragma.
Female. Unknown, difficult to identify except by extrapolation from male siblings or progeny.
Distribution. Known only from Upolu, Samoa: common at the type locality at Malololelei (c. 450 m), rare at Apia (sea-level).
Etymology. This species is named after Dr Malcolm D. Schug (University of North Carolina, Greensboro), who led two expeditions (with Shelly Gray Smith, Michael M. Marshall and Amanda Killon-Atwood) to northern Australia and islands of the South Pacific to explore genetic structure and historical demography of natural populations of Drosophila ananassae .
Remarks
In 1934 Malloch completed a taxonomic study of the Drosophilidae of Samoa, he reported on ten genera and 27 species, many of them he described as new. He discusses one species (species no. 20, p. 301) in context to D. ananassae . Specimens of this species, he notes, were available from the islands of Upolu, Savaii and Tutuila. On Upolu, Buxton and Hopkins had collected specimens at Apia (the capital of Samoa, at sea level) in August 1924 and at Malololelei (at c. 450 m) on 25 November 1924. When Malloch examined these flies he was confident they were all members of one species conspecific with D. similis that Lamb (1914) had described from the Seychelles. But the name D. similis was preoccupied in the genus Drosophila so Malloch proposed the replacement name D. errans Malloch. Malloch (1934a) also noted that D. similis [= D. errans ] and D. ananassae were treated as synonyms by Duda. On this point he appears to have been unconvinced, and instead he emphasized his certainty that the species in Samoa was the same as the species in the Seychelfles, leaving open the question of whether or not it was conspecific with D. ananassae from Ambon and elsewhere in southeast Asia. Malloch apparently did not dissect the male terminalia of any specimens, but he did describe the sex combs ( Malloch, 1933): “The two basal segments of the fore tarsi in [males] have the ventral setulae arranged in transverse series that are quite conspicuous when seen transversely and as well figured by Lamb” ( Lamb, 1914, plate XX, fig. 33; Malloch, 1933). The specimens in the USNM determined as D. errans by Malloch from Malololelei, should be re-examined—it is probable they are D. schugi . There is little doubt that D. similis Lamb is correctly synonymized with D. ananassae Doleschall (Cariou et al., 2008) therefore we do not need to consider the possibility that the similisreplacement name errans may instead be the appropriate name for the species newly described here as D. schugi . Some or all of the Samoan specimens determined as D. errans by Malloch may indeed turn out to be D. schugi but this will have no substantive bearing on the taxonomy proposed here.
Harrison (1954) reported taking large numbers of D. ananassae from Upolu (Vailima and Malololelei), they too should be re-examined because we now know that three species of the complex exist on the island of Upolu: D. ananassae , D. pallidosa and D. schugi . Wheeler & Kambysellis (1966) also refer to “ D. ananassae ” specimens from Upolu noting that the pale and dark forms are probably different species—they were correct.
The genetic data presented by Schug et al. (2007, fig. 3) clearly shows that 23 of the 25 genotypes sampled at Apia and 11 genotypes derived from Malololelei flies are not drawn from the same population (i.e. the same species). There is evidence that 2 of the 25 genotypes from Apia are very closely related to the Malololelei genotypes. We conclude that D. schugi is more common at, but not restricted to, the higher elevation locality at Malololelei, it also occurs at low frequency (based on genetic evidence, ratio is 2:25) at sea level at Apia. The reverse is true: specimens that correspond to D. pallidosa (pale with low sex comb scores and D. ananassae -like male terminalia) are common at Apia and rare, but not absent, at Malololelei (among large numbers of specimens preserved in alcohol collected from Malololelei in 2003, we have detected some that have no sex combs on the third tarsal segment, a condition typical of D. pallidosa and D. ananassae , but because the specimens are pale we have determined them to be D. pallidosa ).
Hybridization tests
A crossing experiment was designed to investigate hybrid fertility between D. pandora , D. anomalata and D. ananassae . Drosophila pandora and D. ananassae isofemale lines were derived from individuals collected at Lake Placid in 2011. Drosophila anomalata iso-female line CHC221 was collected near Deeragun in April 2014 and iso-female lines A5, A25, A29, A41 and A43 were collected from Lake Placid in November 2014.
Under laboratory conditions all pair-wise combinations of the three species— D. ananassae , D. pandora and D. anomalata —will mate. Of the nine possible pair-wise combinations all produce fertile F 1 progeny. However, the degree of F 1 fertility varies greatly. For example, of the three experiments, only one out of 60 crosses between D. pandora female and D. ananassae male produced fertile F 1 ( Tables 3–5). In the reciprocal only 3 of the 60 yielded fertile F 1.
Drosophila ananassae and D. anomalata mate readily in both directions. Of the 320 single-pair crosses, in both directions, about 65% produce viable F 1. These first generation hybrids when inter-crossed with each other are nearly always fertile ( Tables 3–5).
Drosophila pandora crossed with D. anomalata produces significantly fewer hybrids, about 30% of all hybridizations are viable and fertile.
The D. ananassae and D. pandora cross is the least successful, only about 10% are viable.Almost 100% sterility of F 1 hybrids occurs when D. pandora females are crossed with D. ananassae males. Conversely, in the reciprocal cross, on the rare occasion a hybrid is produced, it is always fertile.
Clearly these three species, which occur sympatrically in northern Queensland, warrant further study. The cause of the hybrid sterility is unknown, and an attempt has been made to address the complications that arise with Wolbachia infection by treating with tetracycline. The results in Table 4 demonstrate that Wolbachia has minimal impact and that cytoplasmic incompatibility is not responsible, in this case, for the observed sterility.
Male courtship behaviour
Working with live strains has allowed us to make some preliminary observations of courtship behaviour. Males of the three species D. ananassae , D. pandora and D. anomalata have distinctly different courtship behaviour. Most notably, bobbing behaviour of the D. anomalata male when in front of the female has been observed—a similar behaviour was described by Spieth (1966: 137) in his observation of strains from New Guinea and Queensland. We have been unable to locate specimens from those strains. Spieth also noted “striking differences in the wing displays of courting D. pallidosa and D. ananassae ” (Spieth, 1966; Futch, 1973).
It was noticed during the rearing of D. ananassae and D. pandora that the third instar larvae behave differently just prior to pupation. While D. ananassae prefers to pupate on a piece of card placed in the rearing vial, or on the glass walls of the vial—particularly higher up, D. pandora third instar larvae tend to pupate only on the paper card. When a card is not provided, third instar D. ananassae larvae prefer to pupate above the surface of the culture medium, while third instar D. pandora larvae tend to remain below the surface of the culture medium with only the anterior spiracles exposed.
72 73 74
75 76 77
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |