Macrobiotus cf. recens Cuénot, 1932
|
publication ID |
https://doi.org/ 10.5852/ejt.2018.452 |
|
publication LSID |
lsid:zoobank.org:pub:76171A51-C284-455F-9504-C4CCEFE42D9D |
|
DOI |
https://doi.org/10.5281/zenodo.3815701 |
|
persistent identifier |
https://treatment.plazi.org/id/03C987FC-901D-6C3B-D416-DF03EE78FAD4 |
|
treatment provided by |
Valdenar |
|
scientific name |
Macrobiotus cf. recens Cuénot, 1932 |
| status |
|
Macrobiotus cf. recens Cuénot, 1932 View in CoL
Figs 8–14 View Fig View Fig View Fig View Fig View Fig View Fig View Fig , Tables 7–8
Material examined (114 animals, 48 eggs)
SPAIN: Canary Islands, Gran Canaria, Presa de Lugarejos, 28°02′38″ N, 15°40′22″ W, 885 m a.s.l., lichen on a stone wall. Specimens mounted on microscope slides in Hoyer’s medium (87 animals + 38 eggs), fixed on SEM stubs (8+10), processed for DNA sequencing (4 animals) and used for acetoorcein staining (15 animals). Slide depositories: 87 animals (slides: ES.006.*, where the asterisk can be substituted by the following numbers 1–4, 10–12, 14–17) and 38 eggs (slides: ES.006.*: 5–9, 13) (IZiBB).
Description of the population from Gran Canaria
Animals (measurements and statistics in Table 7)
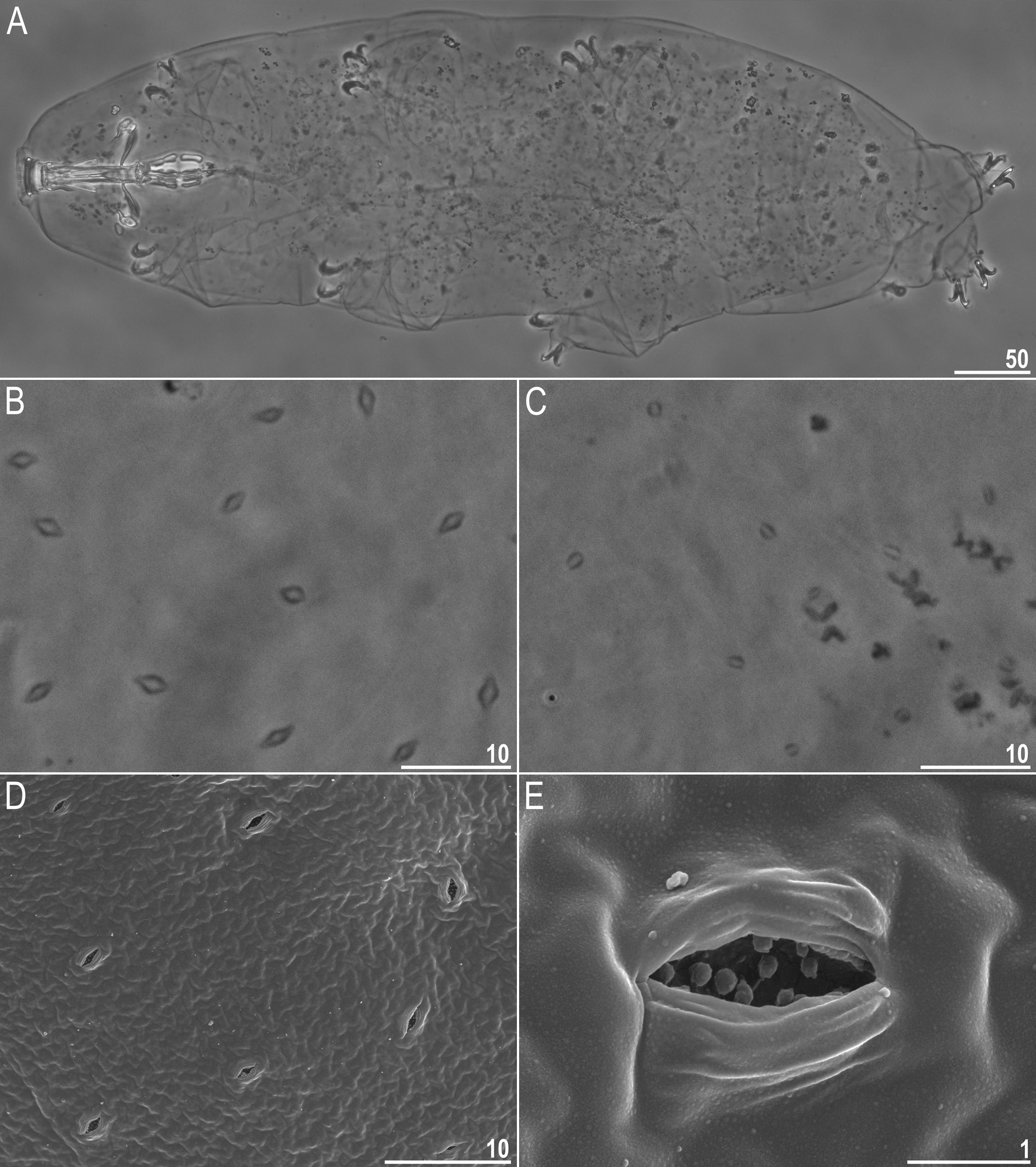
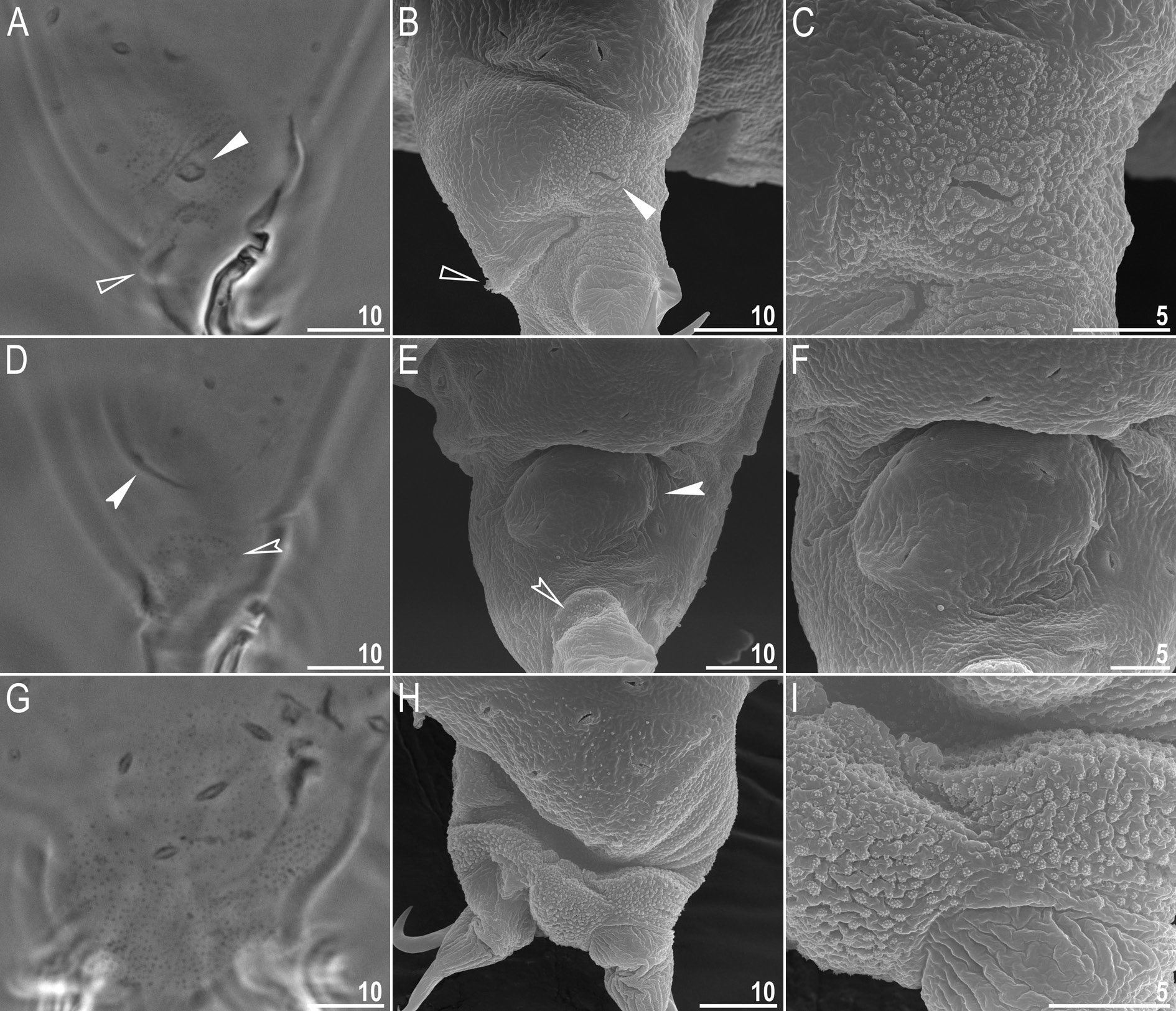
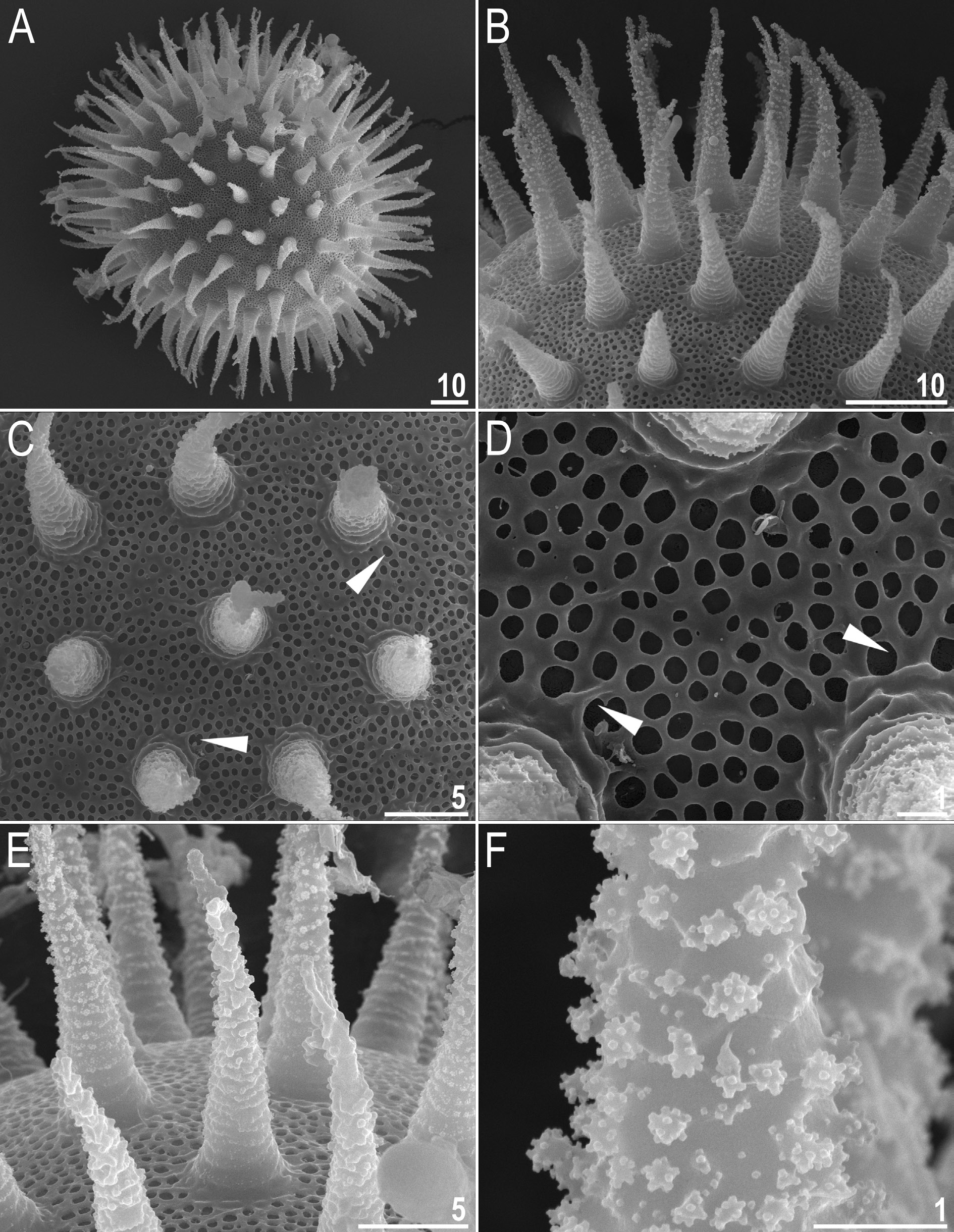
Body white in juveniles and slightly yellowish in adults, after fixation in Hoyer’s medium transparent ( Fig. 8A View Fig ). Eyes present in live animals and in specimens mounted in Hoyer’s medium. Elliptical and sometimes roundish pores (1.0–1.8 μm in diameter), visible under PCM and SEM, scattered randomly on entire body cuticle ( Fig. 8 View Fig B–E), including the external and internal surface of all legs ( Fig. 9 View Fig A–I). Inside pores several granules, visible only under SEM, always present ( Fig. 8E View Fig ). Granulation patch on external and internal surfaces of legs I–III present ( Fig. 9 View Fig A–E). Single pore present at centre of each external granulation patch ( Fig. 9 View Fig A–C). Granulation patch on external surface larger and more distinct than the one on internal surface ( Fig. 9 View Fig A–E). Faint cuticular fold present on external surface of legs I– III just above claws ( Fig. 9 View Fig A–B, empty arrowhead), whereas on internal surface of legs I–III there is a cuticular bulge resembling pulvinus ( Fig. 9 View Fig D–E, filled arrowhead). Both external fold and internal bulge visible only if legs are fully extended and correctly oriented on slide (particularly cuticular fold above claws). Granulation on legs IV always clearly visible and consists of two granulation patches: the distal patch with densely distributed granules situated just above claws and the proximal patch being wider with more sparsely distributed granules located immediately above distal patch ( Fig. 9 View Fig G–I).
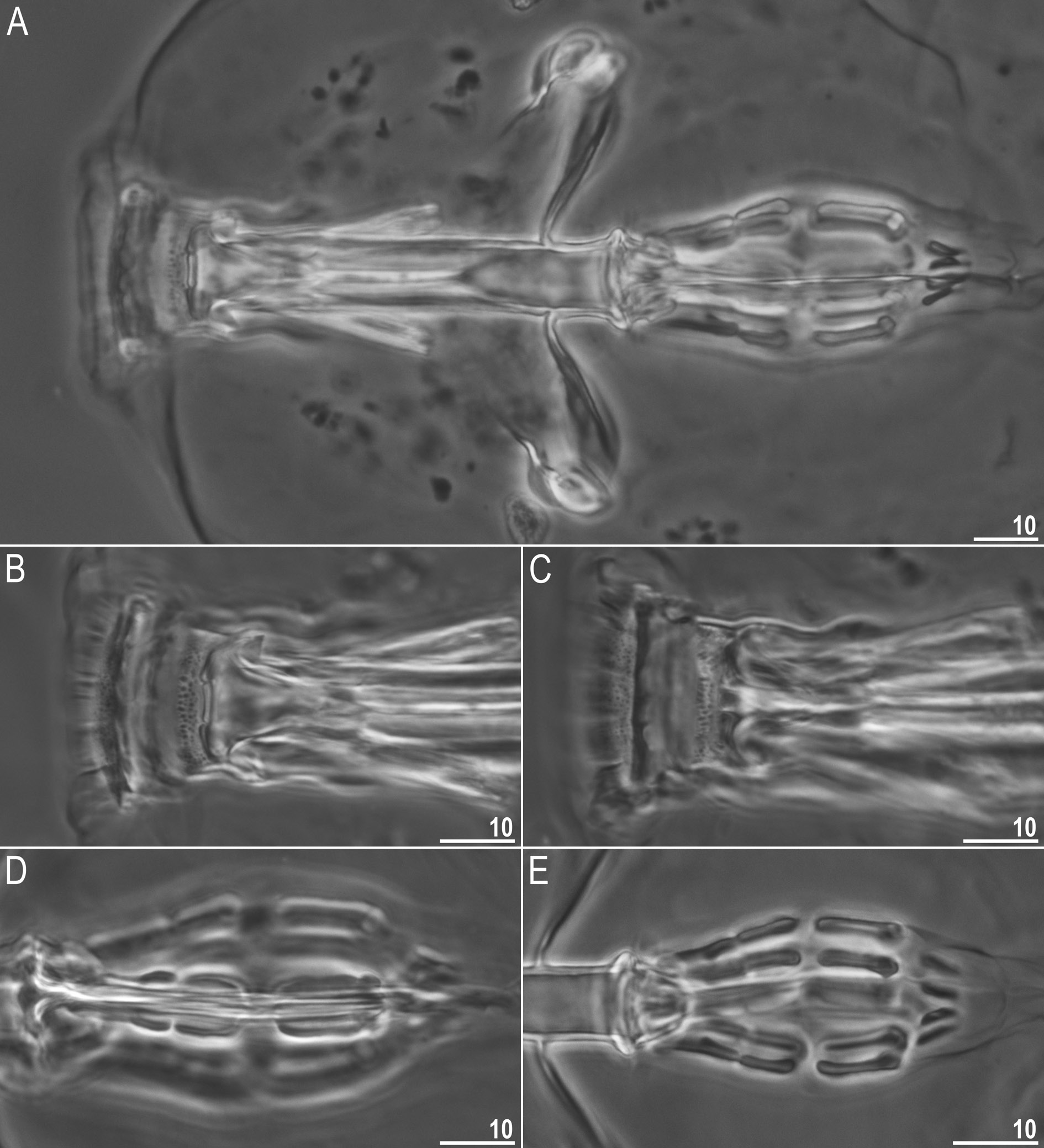
Mouth antero-ventral. Bucco-pharyngeal apparatus of the Macrobiotus type ( Fig. 10 View Fig A–C), with ventral lamina and ten small peribuccal lamellae followed by six buccal sensory lobes. Under PCM, oral cavity armature of the hufelandi type, i.e., with all three bands of teeth always visible ( Fig. 10 View Fig B–C). First band of teeth composed of numerous very small cones arranged in four to six rows situated anteriorly in oral cavity, just behind bases of peribuccal lamellae ( Figs 10 View Fig B–C, 11A–B, filled arrowhead). Second band of teeth situated between ring fold and third band of teeth and comprises 4–5 rows of small cones, slightly larger than those of first band ( Figs 10 View Fig B–C, 11A–B, empty arrowhead). Teeth of the third band located within posterior portion of oral cavity, between the second band of teeth and buccal tube opening ( Figs 10 View Fig B–C, 11A–B). Third band of teeth discontinuous and divided into dorsal and ventral portions. Under PCM, dorsal teeth seen as three distinct transversal ridges, whereas ventral teeth appear as two separate lateral transversal ridges and a roundish median tooth ( Fig. 10 View Fig B–C). Under SEM, both dorsal and ventral teeth clearly distinct ( Fig.11 View Fig A–B). Medio-ventral tooth rarely divided into two or three smaller teeth ( Fig. 11B View Fig ). Under SEM, margins of dorsal and latero-ventral teeth slightly serrated ( Fig. 11 View Fig A–B). Pharyngeal bulb spherical, with triangular apophyses, two rod-shaped macroplacoids and one big rod-shaped microplacoid ( Fig. 10 View Fig D–E). Macroplacoid length sequence 2 <1. First and second macroplacoid with the central and the subterminal constriction, respectively ( Fig. 10 View Fig D–E).
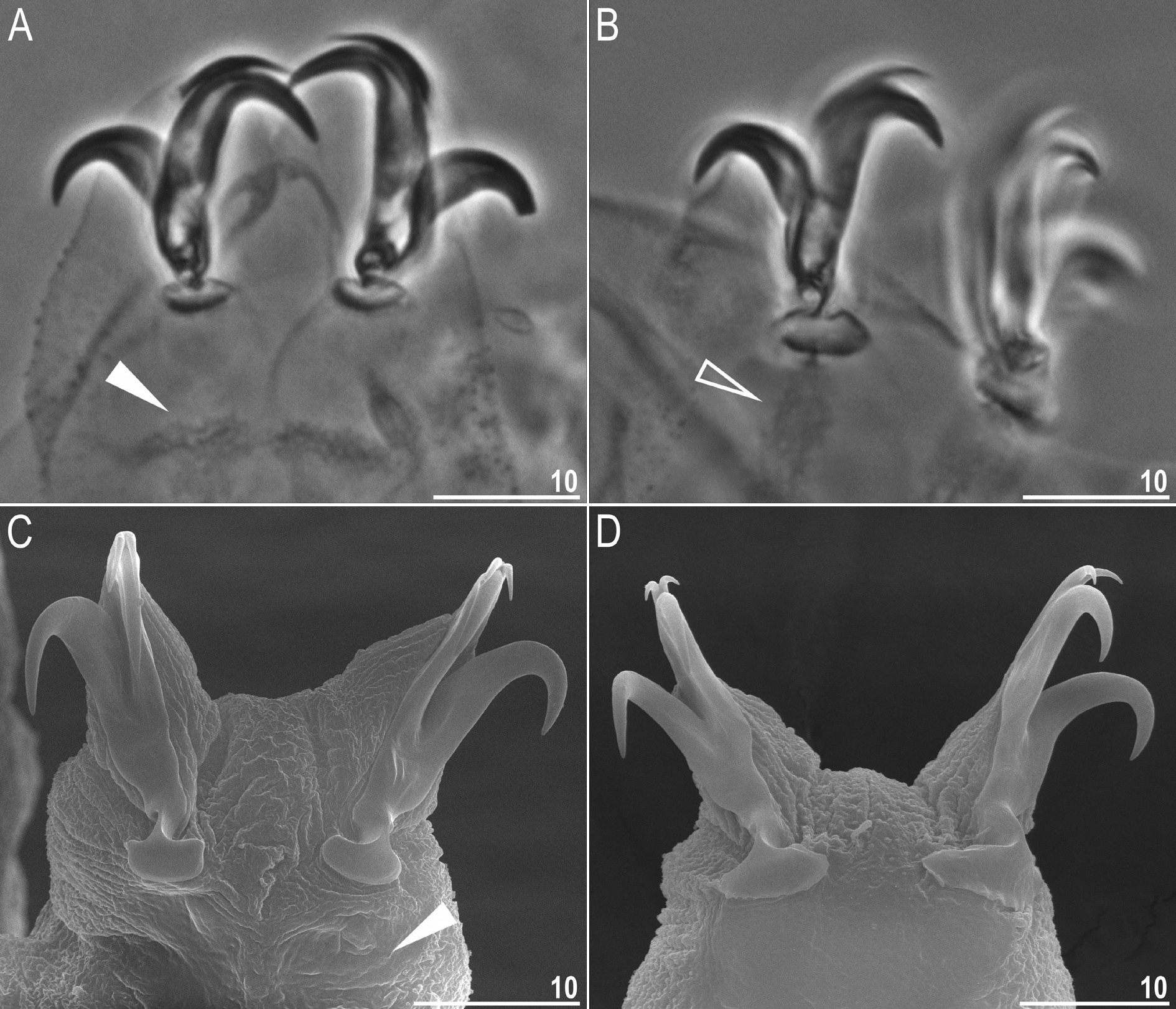
Claws Y shaped, of the hufelandi type ( Fig. 12 View Fig A–D). Primary branches with distinct accessory points and with an evident stalk connecting claw to lunula ( Fig. 12 View Fig A–D). Lunulae I–III smooth ( Fig. 12A, C View Fig ), whereas lunulae IV slightly crenulated ( Fig. 12B, D View Fig ). Faint cuticular bars under claws I–III present, more visible in larger specimens ( Fig. 12A, C View Fig ). Horseshoe-shaped structure connects anterior and posterior lunules ( Fig. 12B View Fig ).
Eggs (measurements and statistics in Table 8)
Laid freely, white/light yellow, spherical or slightly oval ( Figs 13 View Fig A–B, 14A). Surface between processes of the hufelandi type, i.e., chorion surface between processes covered by reticulum with very small meshes ( Figs 13 View Fig C–D, 14B–E). Under PCM, surface between processes seems to be covered by dark dots ( Fig. 13C View Fig ) and only sometimes a clear reticulation is visible ( Fig. 13D View Fig ). Several rows of meshes between egg processes (usually 7–8). Mesh borders and nodes/knots thick and sometimes wider than mesh diameter ( Fig. 14 View Fig B–E). Meshes circular or slightly oval (0.4–0.9 μm in diameter) and under SEM all pores empty inside ( Fig. 14 View Fig C–D). Mesh diameter decreases gradually from peribasal to interbasal meshes ( Fig. 14 View Fig C–D). Short thickenings radiating from process bases are often visible under PCM and always visible under SEM; thickenings may create a crown around process bases ( Figs 13C View Fig , 14 View Fig C–D, filled arrowheads). Processes in the shape of high and thin cones with straight conical trunks, devoid of terminal discs and sometimes bifurcated ( Figs 13 View Fig A–F, 14A–F). Trunks of processes slightly undulated. Undulations covered by numerous granules composed of microgranule aggregations. Undulations and granules poorly visible under PCM ( Fig. 13 View Fig C–F). Undulations, granules and microgranules always clearly visible under SEM ( Fig. 14B View Fig , E–F).
Reproductive mode
The examined population is dioecious (gonochoristic). Males were identified using aceto-orcein staining, which revealed testicles filled with spermatozoa. However, no morphological secondary sexual dimorphism, such as gibbosities on hind legs in males, was identified.
DNA sequences
We obtained sequences for all four of the above-mentioned molecular markers. The two conservative nuclear markers (18S rRNA, 28S rRNA) were represented by single haplotypes, whereas both ITS-2 and COI exhibited two haplotypes. The p-genetic distance between the ITS-2 as well as between the COI haplotypes was 1.1%. The 18S rRNA sequence (GenBank: MH063927 View Materials ) was 1033 bp long, the 28S rRNA sequence (GenBank: MH063936 View Materials ) was 725 bp long, the ITS-2 haplotype 1 and 2 sequences (GenBank: MH063932 View Materials and MH063933 View Materials , respectively) were 420 bp long; the COI haplotype 1 and 2 sequences (GenBank: MH057768 View Materials and MH057769 View Materials , respectively) were 658 bp long.
Genotypic differential diagnosis
The ranges of uncorrected genetic p-distances between the new species and species of the Macrobiotus hufelandi complex, for which sequences are available from GenBank, are as follows:
• 18S rRNA: 0.4–4.0% (2.0% on average), with the most similar being M. macrocalix from Poland ( MH063926 View Materials ) and the least similar being M. polypiformis from Ecuador ( KX810008 View Materials )
• 28S rRNA: 1.3–13.6% (6.5% on average), with the most similar being M. macrocalix from Poland ( MH063935 View Materials ) and the least similar being M. polypiformis from Ecuador ( KX810009 View Materials )
• ITS-2: 3.7–26.7% (16.9% on average), with the most similar being M. macrocalix from Poland ( MH063931 View Materials ) and the least similar being M. polypiformis from Ecuador ( KX810010 View Materials )
• COI: 16.4–24.7% (19.1% on average), with the most similar being M. macrocalix from Italy ( FJ176203 View Materials –7 and FJ176213 View Materials –7) and the least similar being M. papei from Tanzania ( MH057763 View Materials )
Molecular phylogeny
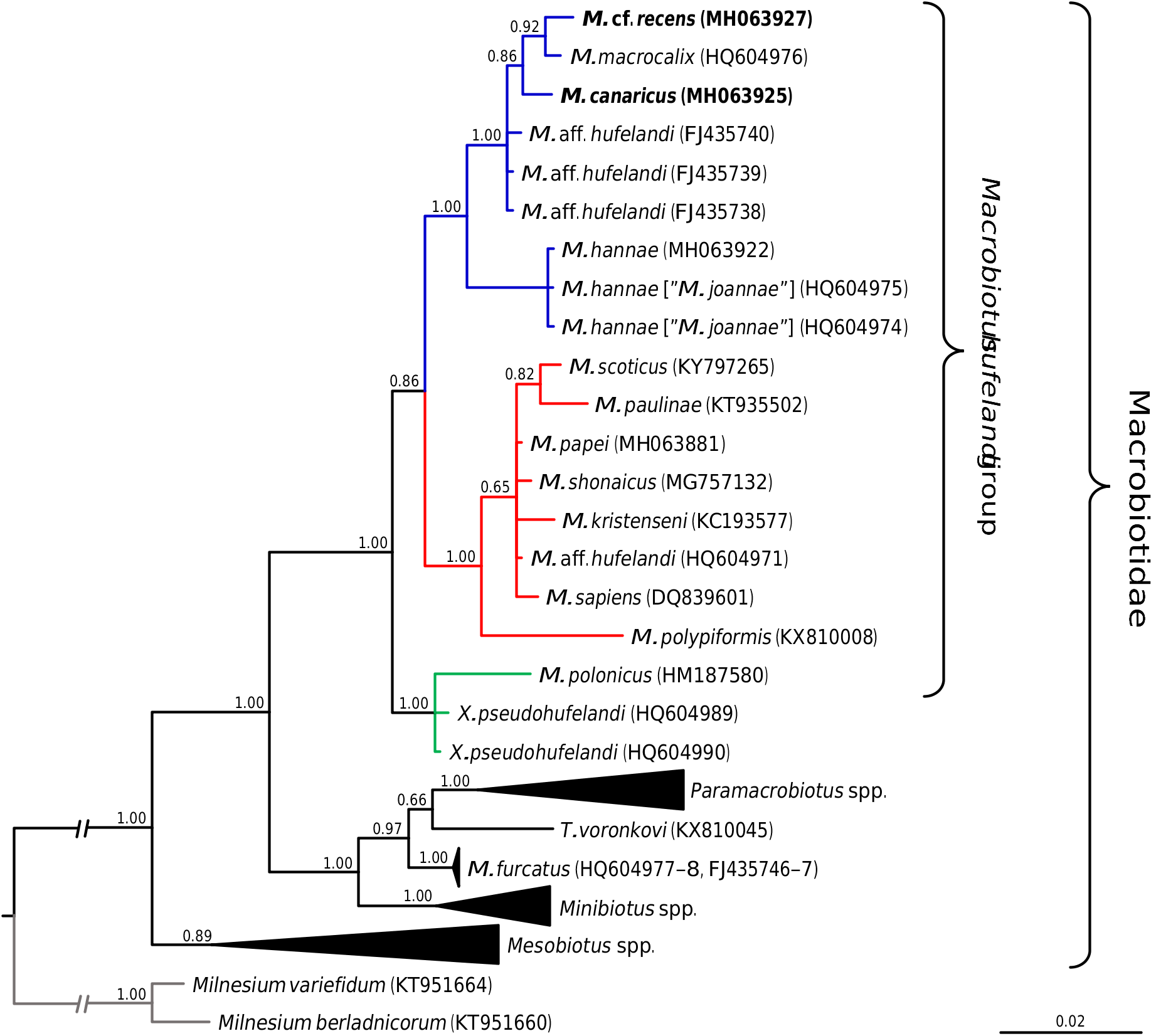
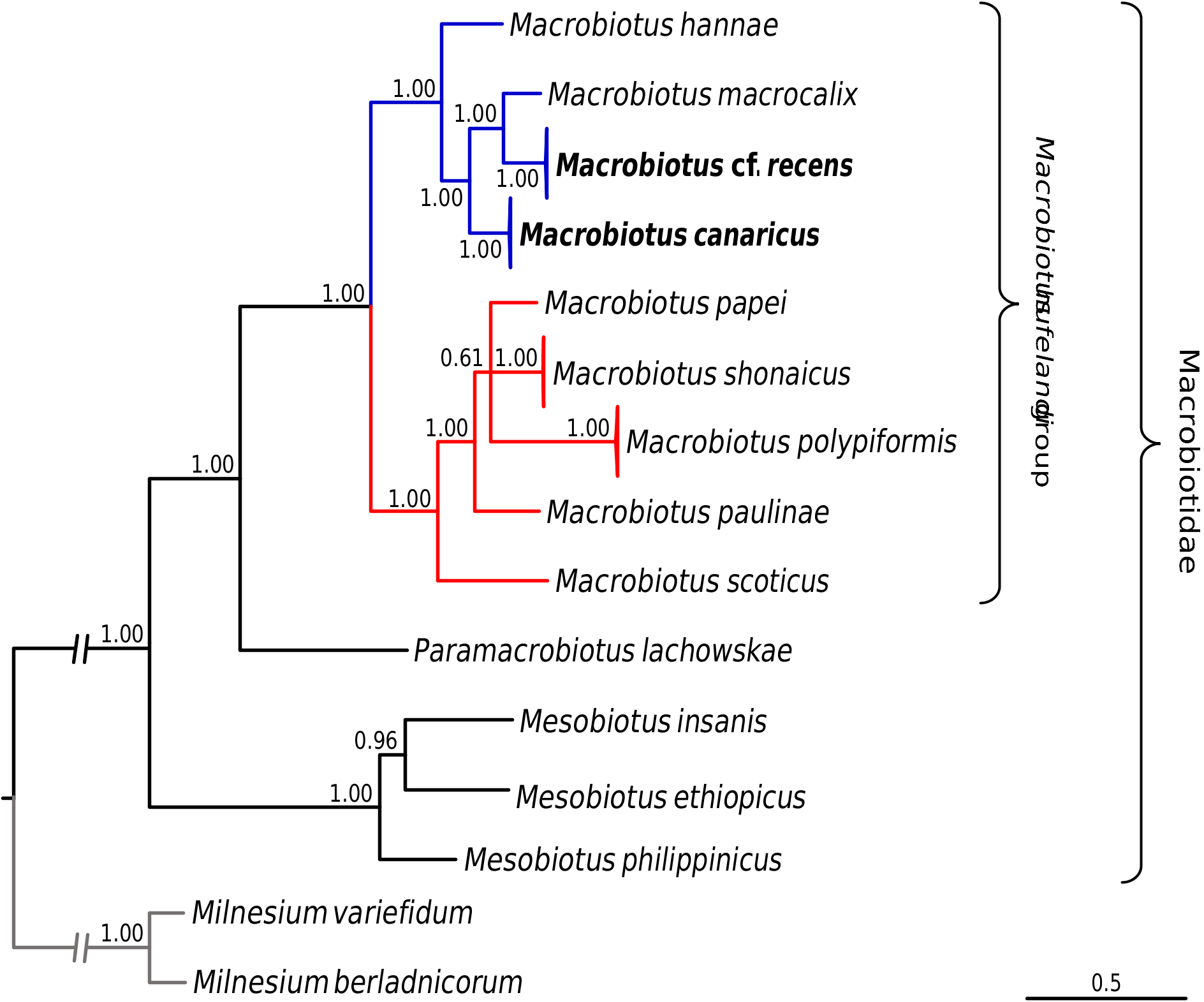
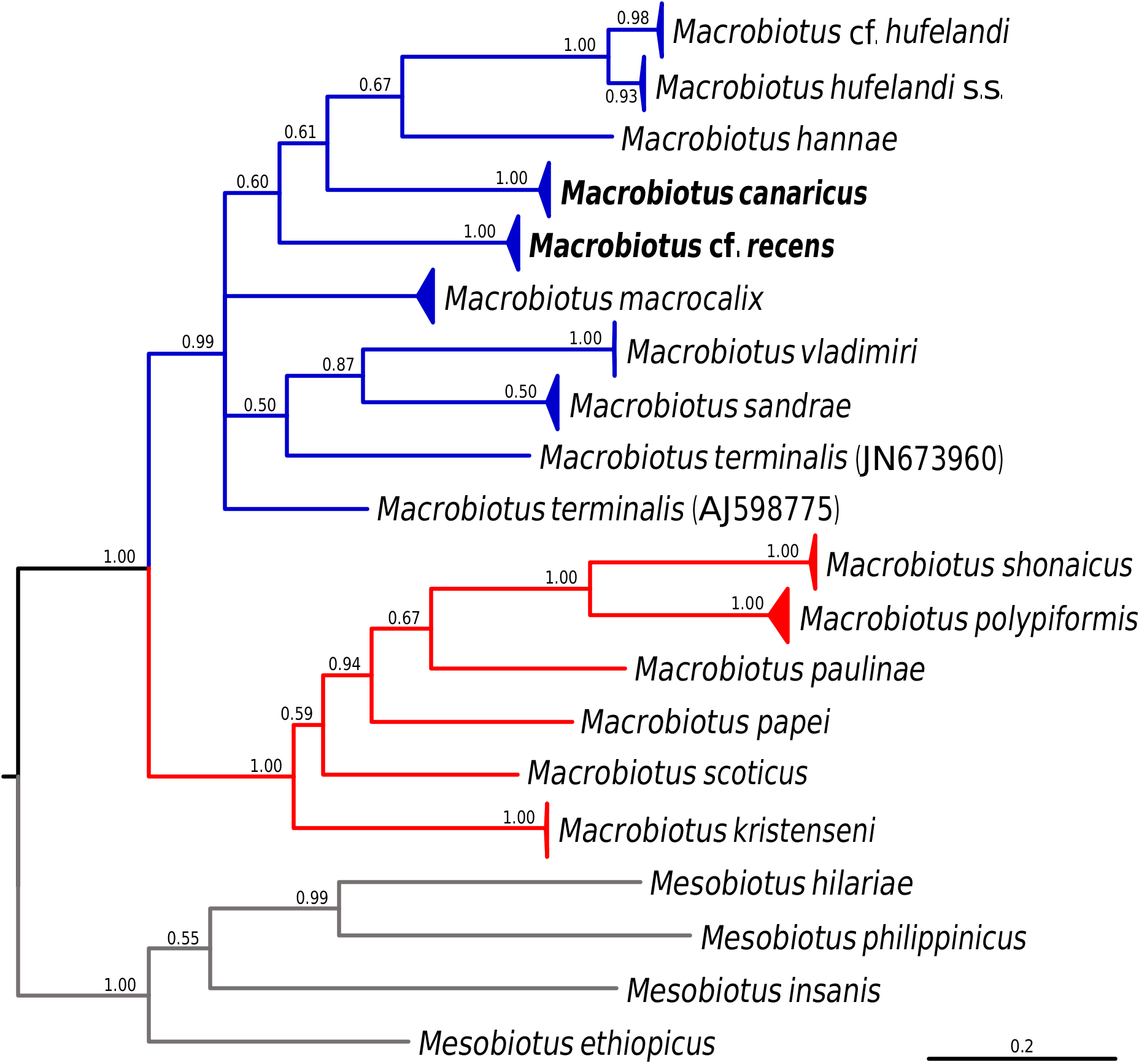
Phylogenetic analyses conducted on macrobiotid 18S rRNA sequences as well as on the concatenated macrobiotid data set unambiguously confirmed that the two studied species represent the M. hufelandi group ( Figs 15–16 View Fig View Fig ). The phylogeny based on the COI sequences of the hufelandi group also corroborated these results, since none of the two species were recovered external to the species of the hufelandi group ( Fig. 17 View Fig ). In all analyses, two clades within the hufelandi group were present, although the species composition varied slightly between phylogenies based on different markers. One clade grouped exclusively species that exhibit modified egg processes ( M. paulinae Stec et al., 2015 , M. polypiformis , M. papei , M. shonaicus Stec et al., 2018a , M. scoticus Stec et al., 2017b and M. kristenseni Guidetti, Peluffo, Rocha, Cesari & Moly de Peluffo, 2013 ); ‘the kristenseni clade’ henceforth. The other clade comprised mostly species with typical inverted goblet-shaped egg processes (‘the hufelandi clade’ hereafter). In contrast to our predictions, M. cf. recens , with its atypical egg processes, was always embedded within the hufelandi clade. The two clades were well supported in phylogenies based on the concatenated data set and on COI sequences, but weakly supported in the 18S rRNA tree ( Figs 15–17 View Fig View Fig View Fig ). Moreover, in the 18S rRNA analysis the kristenseni clade, in addition to the majority of species with modified egg processes, comprised M. sapiens Binda & Pilato, 1984 ( DQ839601 View Materials ) and undetermined species of the M. hufelandi group ( HQ604971 View Materials ), of which at least the first species exhibits the typical egg morphology. In contrast to other analyses, the 18S rRNA phylogeny recovered a clade, with X. pseudohufelandi (Iharos, 1966) and M. polonicus Pilato, Kaczmarek, Michalczyk & Lisi, 2003 , that was in a sister relationship to all other species of the hufelandi group, suggesting that the hufelandi group is polyphyletic or that Xerobiotus belongs to the hufelandi group ( Fig. 15 View Fig ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |