Macrobiotus papei, Stec & Kristensen & Michalczyk, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4446.2.7 |
|
publication LSID |
lsid:zoobank.org:pub:78204F54-9DF7-4807-84A4-6954F4197AA0 |
|
DOI |
https://doi.org/10.5281/zenodo.5963664 |
|
persistent identifier |
https://treatment.plazi.org/id/196C5B1C-784F-FF8B-FF5D-FC0EB3F771E4 |
|
treatment provided by |
Plazi |
|
scientific name |
Macrobiotus papei |
| status |
sp. nov. |
Macrobiotus papei View in CoL sp. nov.
( Tables 3–4, Figures 1–7 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 )
Material examined: 131 animals (including 10 specimens in simplex stage), and 81 eggs. Specimens mounted on microscope slides in Hoyer’s medium (49 animals + 66 eggs), fixed on SEM stubs (20+15), and processed for DNA sequencing (6+0), aceto-orcein staining (50+0), and single individual culturing (6+0).
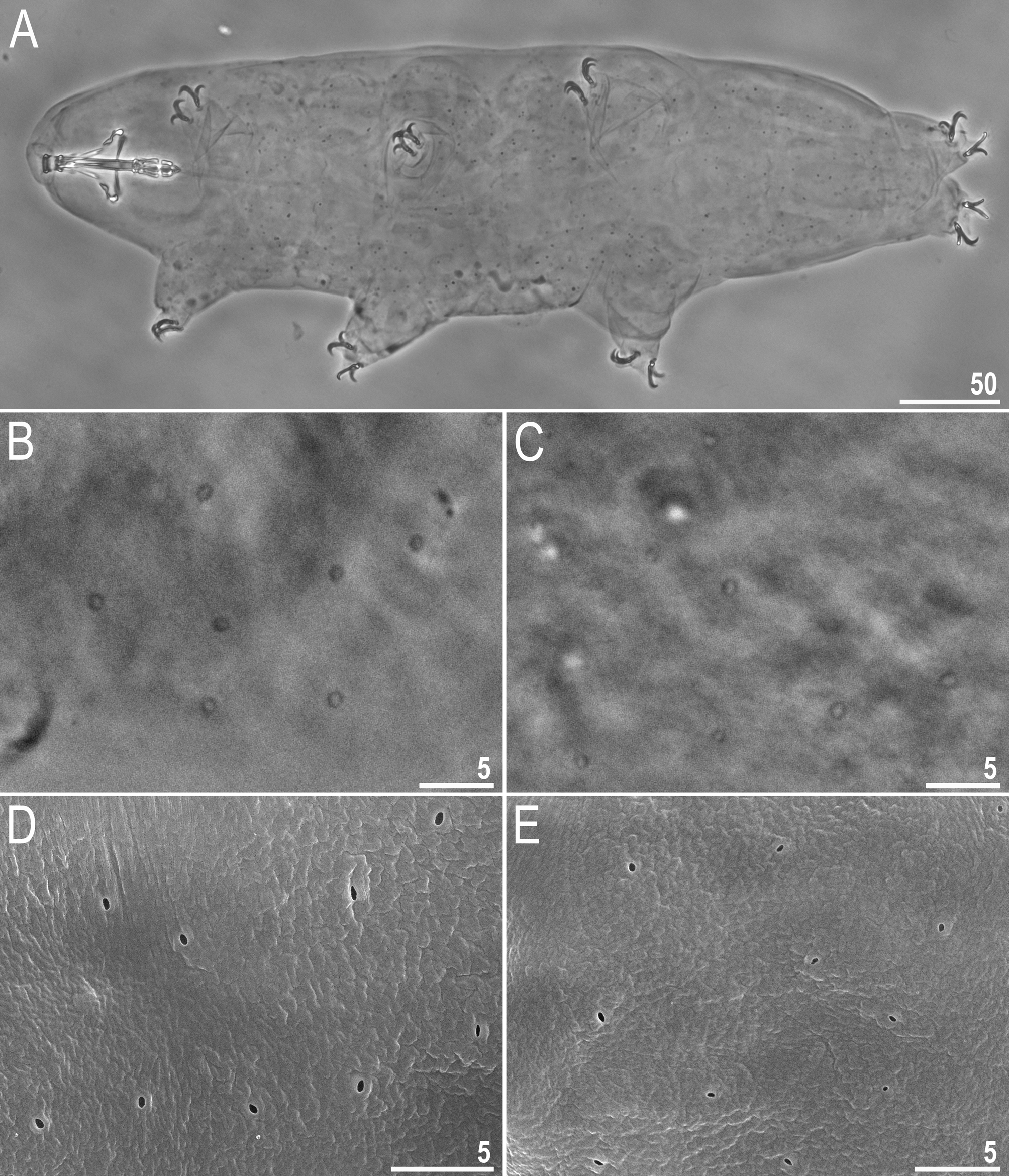
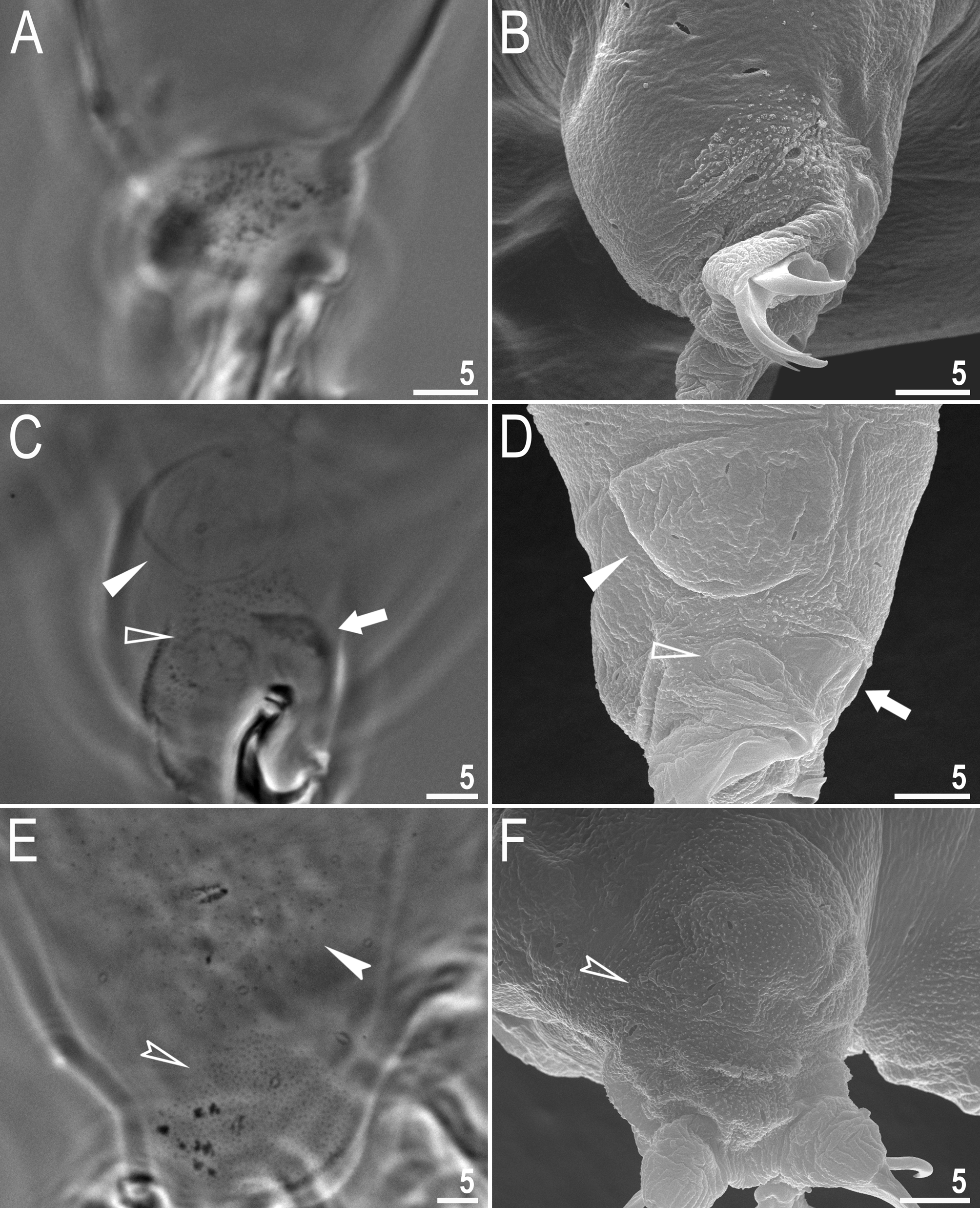
Description of the new species. Animals (measurements and statistics in Table 3). Body white in juveniles and slightly yellowish in adults, after fixation in Hoyer’s medium transparent ( Fig. 1A View FIGURE 1 ). Eyes present in all live animals (dissolved in 80% of specimens mounted in Hoyer’s medium). Small round and oval pores (0.6–1.1 µm in diameter), visible under PCM and SEM, scattered randomly over the entire body cuticle ( Fig. 1B–E View FIGURE 1 ), including the external and internal surface of all legs ( Fig. 2B–E View FIGURE 2 ). Two cuticular granulation patches, one on the external and the other on the internal surface, are present on legs I–III ( Fig. 2A–D View FIGURE 2 ). Granulation on the external surface ( Fig. 2A–B View FIGURE 2 ) is bigger and more distinct than the on the internal surface ( Fig. 2C–D View FIGURE 2 ). A cuticular bulge/fold, resembling a pulvinus, is present on the internal surface of legs I–III ( Fig 2C–D View FIGURE 2 , filled arrowhead), whereas a faint cuticular fold is present just above the claws ( Fig. 2C–D View FIGURE 2 , flat empty arrowhead). Both structures are visible only if the legs are fully extended and well oriented on the slide (particularly in the case of the cuticular fold above the claws). Cuticular granulation on legs IV is always clearly visible and consists of two granulation patches: the distal patch with densely distributed granules situated just above the claws and the proximal patch being wider with more sparsely distributed granules located just above the first patch ( Fig. 2E–F View FIGURE 2 ). Sometimes muscle attachments are also visible under claws I–III ( Fig. 2C–D View FIGURE 2 , arrow)
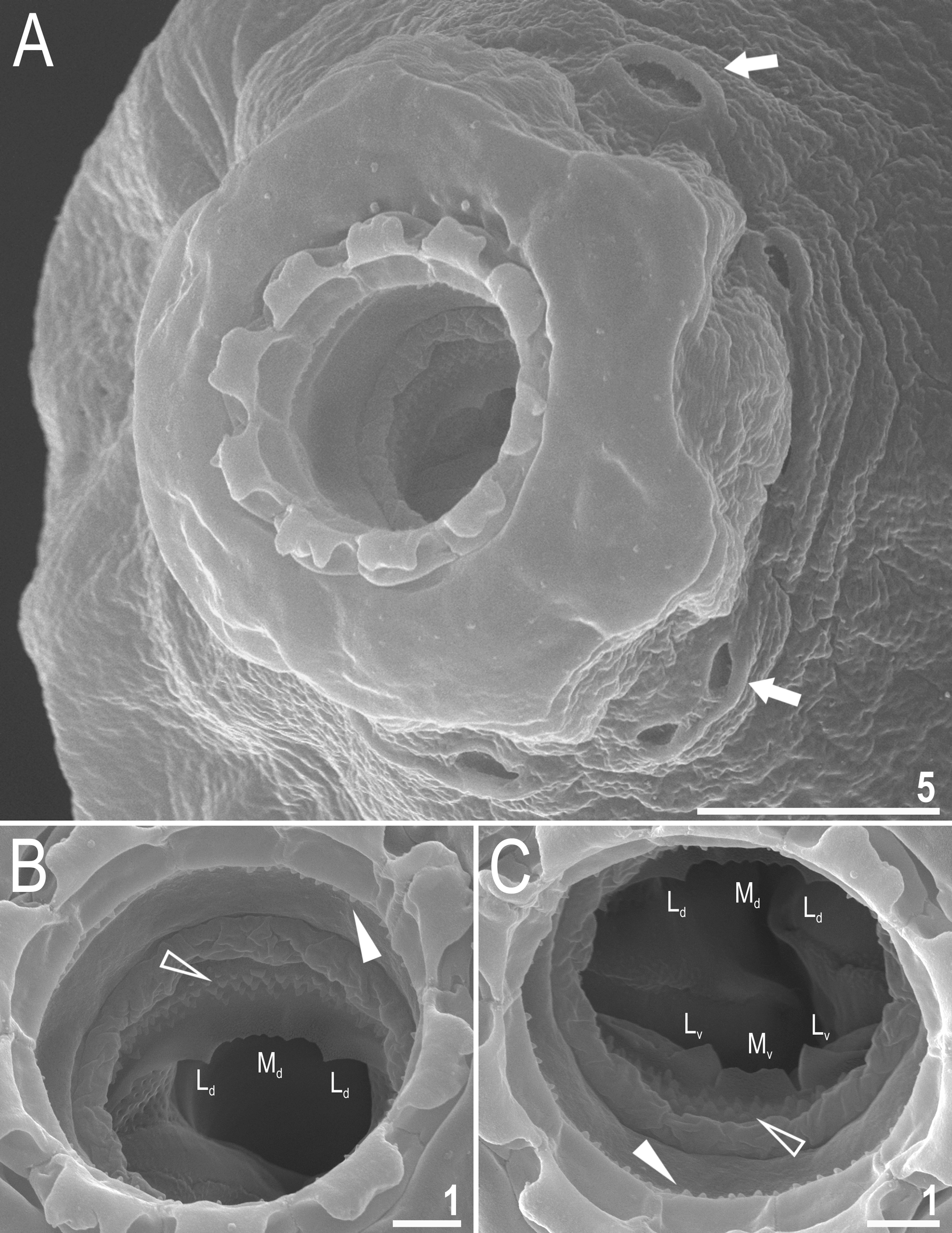
Mouth antero-ventral. Bucco-pharyngeal apparatus of the Macrobiotus type, with the ventral lamina and ten small peribuccal lamellae followed by six buccal sensory lobes ( Figs 3A View FIGURE 3 and 4A–C View FIGURE 4 ). An irregular ring of pores, clearly visible using SEM and occasionally under PCM, is present around the mouth opening, immediately behind the peribuccal sensory lobes ( Figs 3B View FIGURE 3 and 4A View FIGURE 4 , arrow). Under PCM, the oral cavity armature is of the patagonicus type (only the second and third band of teeth visible under light microscopy (LM) in the majority of specimens ( Fig. 3B–C View FIGURE 3 ) but in smaller individuals the oral cavity armature is of the maculatus type (only the third band of teeth visible under LM) ( Fig. 3D–E View FIGURE 3 ). However, under SEM the oral cavity comprises three bands of teeth, i.e. under PCM only the third band or only the second and the third band of teeth are visible ( Fig. 3B–E View FIGURE 3 ), whereas all three bands are always detectable in SEM ( Fig. 4B–C View FIGURE 4 ). The first band of teeth consists of numerous extremely small cones arranged in one to two rows situated anteriorly in the oral cavity, just behind the base of the peribuccal lamellae ( Fig. 4B–C View FIGURE 4 , flat filled arrowhead). The second band of teeth are situated between the ring fold and the third band of teeth and comprises 4–5 rows of small cones, slightly bigger than those of the first band ( Fig. 4B–C View FIGURE 4 , empty flat arrowhead). Under PCM, only the larger teeth of second band are visible in larger specimens and often appear as very weakly developed ( Fig. 3B–C View FIGURE 3 , empty flat arrowhead). The teeth of the third band are located within the posterior portion of the oral cavity, between the second band of teeth and the buccal tube opening ( Fig. 4B–C View FIGURE 4 ). The third band of teeth is discontinuous and divided into a dorsal and a ventral portion. Under PCM, the dorsal teeth form a single transverse ridge with obvious thickenings, whereas the ventral teeth appear as two separate lateral transverse ridges between which a roundish median tooth is visible ( Fig. 3B–E View FIGURE 3 ). The medio-ventral tooth is occasionally divided into two smaller rounded teeth. Under SEM, the dorsal teeth form a single ridge with two larger lateral peaks and several smaller median peaks and indentations ( Fig. 4B View FIGURE 4 ) which correspond to thickenings visible under LM ( Fig. 3B View FIGURE 3 ). The ventral teeth are separated into one median and two lateral teeth ( Fig. 4C View FIGURE 4 ). Pharyngeal bulb spherical, with triangular apophyses, two rod-shaped macroplacoids and a triangular small microplacoid ( Fig. 3A View FIGURE 3 ). The macroplacoid length sequence 2<1. The first and the second macroplacoid are smooth in the majority of specimens, only occasionally with very weak central and subterminal constrictions, respectively ( Fig. 3F–G View FIGURE 3 , arrowheads).
Claws long and slender, of the hufelandi type ( Fig. 5A–D View FIGURE 5 ). Primary branches with distinct accessory points, a long common tract, and with an evident stalk connecting the claw to the lunula ( Fig. 5A–D View FIGURE 5 ). Lunulae I–III smooth ( Fig. 5A, C View FIGURE 5 ), whereas lunulae IV sparsely dentate ( Fig. 5B, D View FIGURE 5 ). Cuticular bars under claws absent.
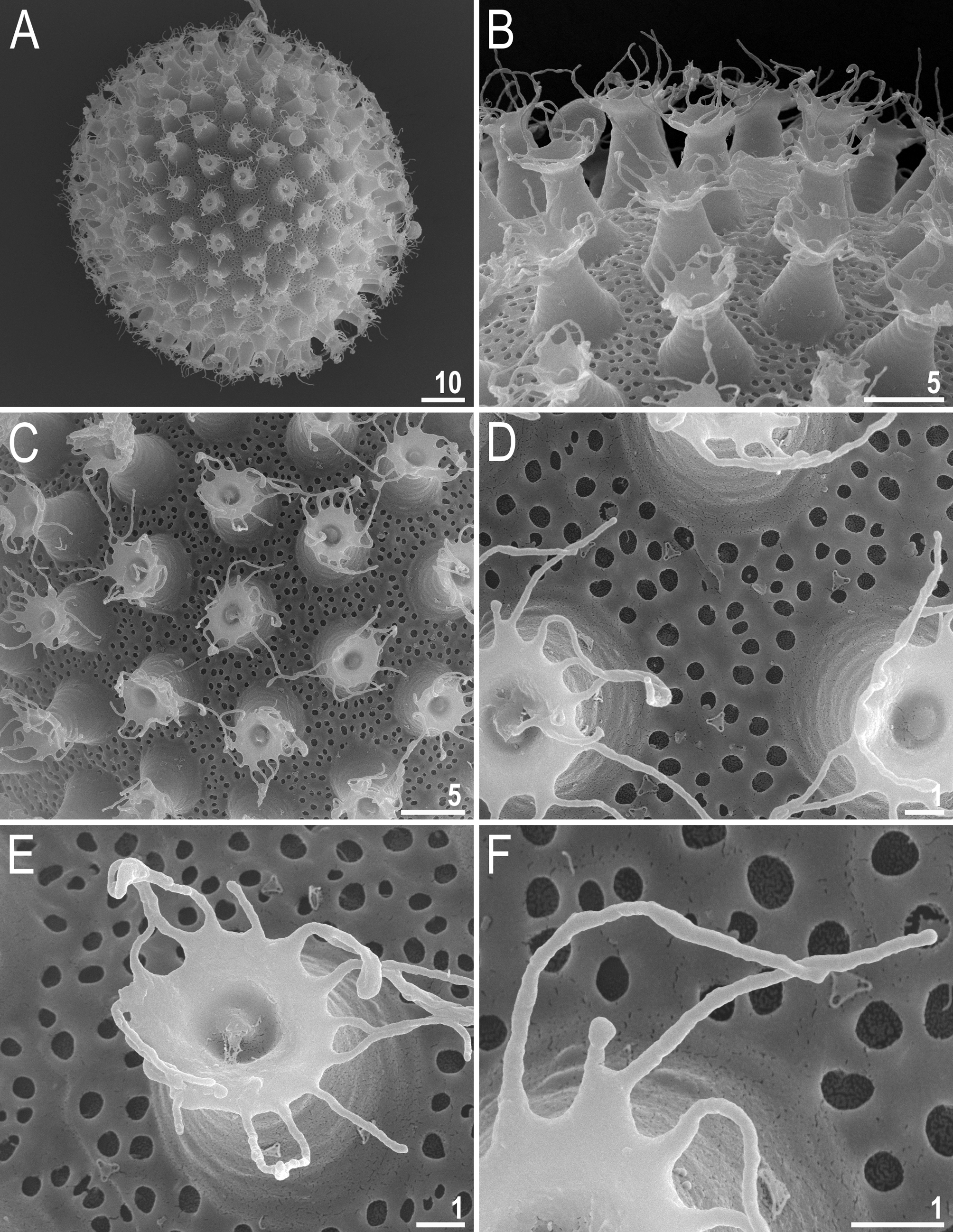
Eggs (measurements and statistics in Table 4). Laid freely, white/light yellow, spherical or slightly oval ( Figs 6A–B View FIGURE 6 and 7A View FIGURE 7 ). The surface between processes of an intermediate type between the hufelandi and the maculatus type, i.e. chorion surface covered numerous small pores that form a reticulum with thick walls ( Figs 6B–D View FIGURE 6 and 7A– F View FIGURE 7 ). Pores are similar in size and uniformly scattered over the entire surface, i.e. there no peribasal rings of elongated pores/bigger mesh around the processes. There are several rows of pores between processes and the mesh bars and nodes are often wider than the pore diameter ( Figs 6C–D View FIGURE 6 and 7B–F View FIGURE 7 ). The pores in the reticulum are circular or slightly oval (0.3–0.6 µm in diameter) and under SEM almost all pores are empty inside ( Fig. 7C–E View FIGURE 7 ). Processes are in the shape of inverted goblets with slightly concave conical trunks and well-defined terminal discs ( Figs 6E–F View FIGURE 6 and 7A–F View FIGURE 7 ). Terminal discs are cog-shaped, with a concave central area and with 10–15 small irregular teeth ( Figs 6E–F View FIGURE 6 and 7B–F View FIGURE 7 ). Almost all teeth on the terminal disc are elongated into thin flexible filaments, less than 0.3 µm in diameter and 2–7 µm in length ( Figs 6E–F View FIGURE 6 , filled arrowhead and 7B–F). The filaments are hair-like under PCM ( Fig. 6E–F View FIGURE 6 ) and under SEM are completely smooth as is the surface of terminal discs ( Fig. 7D–F View FIGURE 7 ). Under PCM, the filaments are clearly visible, however the filaments are very thin and could be overlooked and/or misinterpreted as debris attached to the eggs, if close attention is not paid during examination.
Reproductive mode. Staining 50 adults of the new species with aceto-orcein did not reveal any individuals with a testicle filled with spermatozoa (males), an ovotestis with spermatozoa and oocytes (hermaphrodites), or with spermatozoa in a spermatheca (inseminated females). Only females with oocytes in the ovary or specimens of an undetermined sex were observed. Moreover, both PCM and SEM observations showed no morphological secondary sexual dimorphism such as gibbosities on legs IV (e.g. Rebecchi & Nelson 1998). Furthermore , to test whether females are capable of parthenogenetic reproduction, we isolated eggs and observed the full cycle of hatchlings maturing to adults, laying the next generation of eggs still in isolation, and the emergence of the second generation, also from individually isolated eggs (i.e. there was no possibility of fertilisation). Thus , all lines of evidence show the type population of M. papei sp. nov. comprises only females capable of parthenogenetic reproduction.
DNA sequences. We obtained very good quality sequences for all four molecular markers from all analysed specimens (paragenophores). DNA sequences of all markers were represented by single haplotypes:
The 18S rRNA sequence (GenBank: MH063881 View Materials ), 905 bp long:
TAGATCGTAATTTTACACGGATAACTGTGGTAATTCTAGAGCTAATACGTGCAACCAGCTCGTTCCCTTGTGGAGCGAGC GCAGTTATTAGAACAAGACCAATCCGGCCTTCGGGTCGGTACAATTGGTGACTCTGAATAACCGAAGCGGAGCGCATGGT CTCGTACCGGCGCCAGATCTTTCAAGTGTCTGACTTATCAGCTTGTTGTTAGGTTATGTTCCTAACAAGGCTTCAACGGG TAACGGGGTATCAGGGTCCGATACCGGAGAGGGAGCCTGAGAAACGGCTACCACATCCAAGGAAGGCAGCAGGCGCGCAA ATTACCCACTCCTAGCACAGGGAGGTAGTGACGAAAAATAACGATGCGAGGGCTAATAGCTTCTCGTAATCGGAATGGGT ACACTTTAAATCCTTTAACGAGGATCTATTGGAGGGCAAGTCTGGTGCCAGCAGCCGCGGTAATTCCAGCTCCAATAGCG TATATTAAAGTTGCTGCGGTTAAAAGCTCGTAGTTGGATCTGGGCTTCTGAATGGATGGTTCACTTTACGGTGTAACTGT TCGTTTGGTGCCACAAGCCGGCCATGTCTTGCATGCCCTTTACTGGGTGTGCATGGCGACCGGAACGTTTACTTTGAAAA AATTAGAGTGCTCAAAGCAGGCGTATGGCCTTGCATAATGGTGCATGGAATAATGGAATAGGACCTCGGTTCTATTTTGT TGGTTTTCGGAACTCGAGGTAATGATTAAGAGGAACAGACGGGGGCATTCGTATTGCGGCGTTAGAGGTGAAATTCTTGG ATCGTCGCAAGACGAACTACTGCGAAAGCATTTGCCAAGAATGTTTTCATTAATCAAGAACGAAAGTTAGAGGTTCGAAG GCGATCAGATACCGCCCTAGTTCTA
The 28S rRNA sequence (GenBank: MH063880 View Materials ), 787 bp long:
TACTAAGCGGAGGAAAAGAAACCAACGGGGATGCCGACAGTAACTGCGAGTGAAATCGGCCAAGCCCAGCGCCGAATCCT GTTGCTGGTAACGGTGGTAGGAACTGTGGCGTGAAGAACGTCCTTACCGGTACGGTTTGCGTGCGTAAGTTCTCCTGAGT GAGGCTCCATTCCAAGGAGGGTGCAAGACCCGTATCGCGTGCAACCGGTATCGGTGTAAGATGTTCGGAGAGTCGCCTTG TTTGTGAGTACAAGGTGAAGTCGGTGGTAAACTCCATCGAAGGCTAAATATGACCACGAGTCCGATAGCGAACAAGTACC GTGAGGGAAAATTGAAAAGCACTTTGAAGAGAGAGCGAAACAGTGCGTGAAACCGCTCAGAGGCAAGCAAATGGGGCCTC GAAGGCAAGGCAGCGAATTCAGCTGGTGGTCTGCGTGGCTGGCCGGTTAAGTGATCTTAACGACTCTTGCCGGTTATGTC TAGCGTAGGTGCCAGTGCACTTTCGTTGCTTGTACGCCACCGCCGTTGAGTGGGCATCCGTCGGGTTGGTAACGCGAAGC CTTACGCCTTCACGGGCGTAGGTGCTTGCAGCCGACTTTGTACGCGTTTGCACTTCAACCGGTCATGTTTGCATGTGCCA GCATTTGCGTTGGATTGGCTCGCTCTGCCGTTTGTCGTGAGATGACGAGCTTGCTCGGCTCTTCGGCATCTATGGTAGAC TCGTGTCGGTTTTCAACGTGGGCACATTGTTAATTCGGTGGCGAGTAGATGGCTGCCCATTTAACCC
The ITS-2 sequence (GenBank: MH063921 View Materials ), 397 bp long:
TTTGTGAACGTTAATTCTTCGAACGCACATTGCGGCTTCGGGTTAACTGAAGCCATGCCTGGTTGAGGGTCAGTTGAAGA AAAAAATCGTAATCGCGCATTGATTACGGAGTGTCTGGTTAATGGCTCGTCCGTTTCCAGATGAAGTATAGACCAGATGT GTGCGCTCATTTGACCGGTGCAAGCAACGCTTTGCCGAGTTGGAGCATTCGGCTTTCTTAGCCGTGCGCCGCAGTTGCAC
GATGGCTAAGTTGGCTACCAACAATGGCGAAGTAAGACCGGTTCAGAGGTGCGCAACGCAATAGGCACATCTGTGTACCA AAAAGAACGTACGGTCGCGGTGTTTCGACCGATGCGGACTTAACTCATTCTTTTGACCTCAGCTCAGACAAGATTAC
The COI sequence (GenBank: MH057763 View Materials ), 745 bp long:
ACTGCCTCTGTGGGAACGTCCTTAAGATTTCTAATTCGGAGGGAATTAAGCCAACCAGGGCTTCTTCTCTCCGACGAACA AATATATAATGTGATTGTAACAAGCCACGCATTTATTATAATTTTTTTTTTCGTGATACCAATTTTAATCGGGGGATTCG GGAACTGACTGGTCCCCCTTATAATTAGAGCACCAGACATAGCTTTTCCACGAATAAATAATTTAAGCTTCTGGATACTG CCCCCCTCTTTTTTCCTAATTACTTTAAGCTCGATAACTGAACAGGGGGCCGGAACCGGATGAACTGTTTACCCACCCCT TTCTCATTTTTTTGCTCACAGGGGCCCTAGAGTAGATCTTACAATTTTTTCACTTCATGTGGCGGGAATTTCATCAATTT TAGGGGCTATTAATTTCATTTCTACAATTCTAAATATGCGAGTACCCCACCTAACCTTAGAAAAAATACCTCTCTTTGTG TGATCCGTTTTTTTAACAGCTATTTTATTACTTCTGGCCCTCCCAGTATTGGCAGGAGGTATTACTATGCTATTACTGGA CCGAAACTTTAATACTTCTTTTTTTGACCCTGCGGGGGGGGGAGACCCTATTTTATACCAACACCTATTTTGGTTTTTTG GCCACCCCGAAGTATATATTTTAATCTTACCAGGCTTTGGAATTATCTCACAAATCGTTATTCACTTCAGAGGAAAATCT CTCACATTCGGACATTTAGGAATAA
Type locality: 7°49'25''S, 36°49'32''E; 2050 m asl: Tanzania, Morogoro Region, Udzungwa Mts. National Park, Mwanihana Peak ; lichen on branches of a bush; coll. 16.08.2016 by Thomas Pape. GoogleMaps
Etymology: We take great pleasure in dedicating this new species to the colleague and friend of the first and the second authors, an established taxonomist of Diptera, Thomas Pape, Head of Section of Biosystematics of the Natural History Museum of Denmark, University of Copenhagen.
Type depositories: Holotype (slide TZ.027.01 with 2 paratypes) and 34 paratypes (slides: TZ.027.*, where the asterisk can be substituted by any of the following numbers 02–06, 0 8, 22) and 56 eggs (slides: TZ.027.*: 10–16, 18, 19, 21) are deposited at the Institute of Zoology and Biomedical Research, Jagiellonian University, Gronostajowa 9, 30-387, Kraków, Poland and 12 paratypes (slides: TZ.027.*: 0 7, 09) and 10 eggs (slides: TZ.027.*: 17, 20) are deposited in the Zoological Museum, Natural History Museum of Denmark, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen Ø, Denmark .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |