Allobates trilineatus (Boulenger, 1883)
|
publication ID |
https://doi.org/10.11646/zootaxa.4951.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:37CA98B1-2AFF-4746-A77E-D0BB6F3706C7 |
|
DOI |
https://doi.org/10.5281/zenodo.4681748 |
|
persistent identifier |
https://treatment.plazi.org/id/03D82D22-FFF6-F300-FF1E-FC6B4FDBF83D |
|
treatment provided by |
Plazi |
|
scientific name |
Allobates trilineatus (Boulenger, 1883) |
| status |
|
Allobates trilineatus (Boulenger, 1883) View in CoL
Figs. 4–12 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 View FIGURE 11 View FIGURE 12
Holotype. BMNH 1947.2 .14.20, from Yurimaguas , Huallaga River, Departamento Loreto, Peru.
New material. Sixteen specimens. Eight adult males: MCP 14303–04 View Materials , 14307 View Materials , 14309 View Materials , 14315 View Materials , CRBIIAP 2113, MHNSM 40420–21 View Materials (field codes: SCF 2225–26, 2229, 2231, 2237–2238, 2234, 2232 respectively). Four adult females: MCP 14306, 14311 View Materials , 14317 View Materials , MHNSM 404023 View Materials (field codes: SCF 2228, 2233, 2239, 2227 respectively). Four juveniles: MCP 14301, 14308 View Materials , 14313 View Materials , MHNSM 40422 View Materials (field codes: SCF 2223, 2230, 2235, 2224 respectively). All specimens collected by Giussepe Gagliardi-Urrutia, Andrés Felipe Jaramillo, Lourdes Y. Echevarría, and Santiago Castroviejo-Fisher in February 11, 2019, in Munichis village , municipality of Yurimaguas, province of Alto Amazonas, department of Loreto, Peru ( 5.89963° S, 76.23317° W, 146 m a.s.l) GoogleMaps .
Amended definition. Allobates trilineatus is characterized by the unique combination of the following character states: (1) Small body size ( SVL: 14.6–16.4 mm in females, 14.9–16.6 mm in males); (2) dorsal skin of body shagreened, and moderately granular from mid body to urostyle; small and scattered tubercles on dorsal surface of thigh and shank; ventral skin smooth; (3) snout subacuminate in dorsal and ventral views, and rounded in lateral view; (4) nostrils visible in lateral and ventral views; (5) tympanum small (~ 0.7 times the eye diameter); (6) short teeth present on the maxillary arch under magnification; (7) dentigerous process of vomer absent; (8) lingual process absent; (9) vocal sac in males single; (10) paired dorsal digital scutes present; (11) metacarpal ridge absent; (12) thenar tubercle conspicuous; (13) nuptial excrescences on thumb absent; (14) basal webbing absent on fingers; (15) Finger III swollen on preaxial side in males; (16) Finger II not swollen; (17) tip of Finger IV not reaching distal subarticular tubercle of Finger III; (18) Finger II shorter than Finger I; (19) low and poorly defined lateral keels on preaxial and postaxial sides on all fingers; (20) discs on fingers I– IV moderately expanded (width of discs on fingers I– IV /width of distal phalanges on fingers I– IV: mean = 1.4 ± 0.2, range = 1.3–1.8); (21) black gland absent on arm; (22) tarsal keel present, tubercle-like, strongly curved; (23) tarsal fold absent; (24) metatarsal fold present, swollen or tubercle-like at the proximal portion and flattened distally; (25) basal webbing present between toes III and IV, absent between other toes; (26) low and poorly defined lateral keels on preaxial and postaxial sides on all toes; (27) length of Toe III surpassing the proximal margin of central subarticular tubercle of Toe IV; (28) discs of toes I and II unexpanded to moderately expanded (width of toe discs range 1.0–1.8 and 1.0–1.8 times the width of their adjacent phalanges, respectively); discs of toes III, IV, and V moderately to greatly expanded (width of discs range 1.4–2.5, 1.3–2.5 and 1.3–2.0 times the width of their adjacent phalanges, respectively); (29) background color of dorsum brown, lighter on tip of snout and urostyle region; (30) pale dorsolateral stripe present, straight and well-defined, merging with lighter dorsal color of the urostyle region, not broadening posteriorly to eyelid; (31) background color of dorsal surface of arms dark yellow in life, cream in preserved specimens; (32) dorsal surface of thigh light brown in live and preserved specimens; a transverse dark brown bar can be present on dorsal surface of thigh; (33) dark brown lateral stripe surrounding the whole body, reaching leg-body insertion; (34) pale ventrolateral stripe present in live and preserved specimens, continuous, extending from tip of snout to groin, iridescent white to cream in life; (35) pale paracloacal mark present, round or comma-shaped; (36) gray to dark gray throat in live and preserved males; (37) throat weakly pigmented in live and preserved females, with scattered grayish-brown melanophores present anteriorly; (38) belly with gray melanophores and scattered iridescent white dots in males; (39) belly surface white with scattered iridescent white dots in females, some specimens with light brownish gray mottling; (40) iris golden, with dark brown reticulation; (41) large intestine unpigmented; (42) testis unpigmented; (43) mature oocytes dark brown; (44) habit diurnal, males vocalizing in daytime; (45) advertisement calls characterized by the emission of note-pairs, with a peak frequency of 5.06–5.81 kHz.
Amended description of external morphology. All morphometric measurements overlap in range between female and male specimens ( Table 1 View TABLE 1 ). Body robust. Head slightly longer than wide (HL/ HW = 1.0–1.2 [1.1 ± 0.04] times), head length 0.36–0.45 (0.4 ± 0.02) times the SVL. Snout subacuminate in dorsal and ventral views, and rounded in lateral view. Nostrils located posterolaterally to snout, visible in ventral, lateral and anterior views. Distance between nostrils 0.4–0.5 (0.4 ± 0.03) times the head width. Canthus rostralis slightly convex in cross section. Distance from nostril to anterior corner of eye 0.5–0.7 (0.6 ± 0.05) times shorter than eye diameter. Loreal region straight and oblique. Eye prominent, diameter 0.13–0.18 (0.14 ± 0.05) times the SVL. Tympanum round, small, 0.4–0.5 (0.4 ± 0.04) times the eye diameter. Tympanic annulus visible under magnification. Supratympanic fold absent. Maxillary teeth conspicuous under magnification. Dentigerous processes of vomers absent. Choanae round, small, positioned anteriorly to eye bulge. Tongue longer than wide, with anterior third attached to the mouth floor. Median lingual process absent. Lateral vocal slits conspicuous. Vocal sac single, extending from middle level of throat to chest.
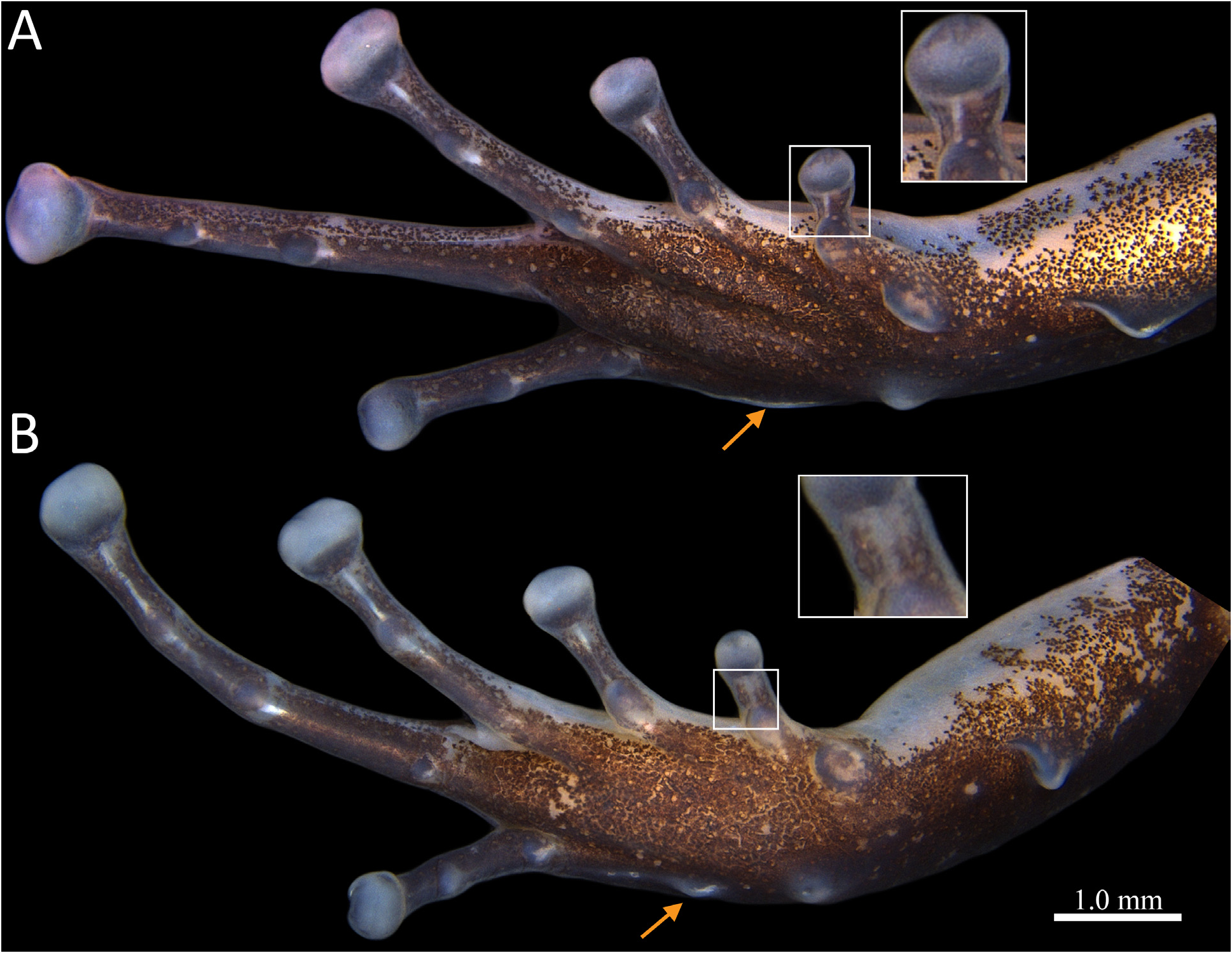
Forelimb slender, ulnar tubercles absent on ventroexternal surface of forearm. Hand length 22.1–28.0 % (25.2 ± 1.6 %) of the SVL. Palmar tubercle conspicuous, round. Thenar tubercle elliptical, maximum diameter 58.3–100 % (75.6 ± 13.0 %) of maximum diameter of palmar tubercle. Proximal subarticular tubercle of Finger IV round, small, not exceeding the width of phalanx. Distal subarticular tubercle absent on Finger IV ( Fig. 4 View FIGURE 4 ). Finger III with a round, protuberant proximal subarticular tubercle and a small, round distal subarticular tubercle. Subarticular tubercles on fingers I and II very protuberant, elliptical. Supernumerary tubercles absent. Metacarpal ridge absent. Webbing absent between fingers. Length of Finger II 88.2–96.7 % (92.7 ± 2.5 %) of Finger I length. Tip of Finger IV not reaching the distal end of distal subarticular tubercle of Finger III when fingers are juxtaposed. Relative lengths of fingers adpressed: IV < II <I < III. Poorly defined keels lateral on the preaxial and postaxial of fingers ( Fig. 4 View FIGURE 4 ). Finger III preaxially swollen in males ( Fig. 4A View FIGURE 4 ), finger swelling expanding from the base of the Finger III disc to the level of proximal subarticular tubercle. Discs of fingers moderately expanded, width of discs I– IV corresponding to 1.2–1.8 (1.5 ± 0.2), 1.1–1.7 (1.4 ± 0.2), 1.3–2.0 (1.7 ± 0.2), and 1.1–1.7 (1.5 ± 0.2) times the width of their adjacent phalanges, respectively. Tip of discs rounded. Paired dorsal digital scutes conspicuous and protuberant in all fingers.
Hind limbs robust. Lengths of thigh, shank, and foot similar (TL/ SVL = 45.3–56.5 [49.2 ± 3.1] %; SAL/ SVL = 47.4–61.6 [51.7 ± 3.7] %; FL/SLV = 36.0–53.0 [47.0 ± 4.2] %). Tarsal keel tubercle-like, short, strongly curved at its proximal end. Inner metatarsal tubercle well-defined, large, elliptical. Outer metatarsal tubercle shorter, round. Metatarsal fold present between outer metatarsal tubercle and the proximal subarticular tubercle of Toe I, swollen or tubercle-like at its proximal portion, flattened distally, or completely flattened ( Fig. 5 View FIGURE 5 ). Basal webbing present between toes III and IV, absent between other toes. Toes slender, all with poorly defined keels on the preaxial and postaxial sides. Subarticular tubercles round, smaller than inner metatarsal tubercle in maximum diameter. Plantar supernumerary tubercles absent. Relative lengths of toes: I < II < V < III < IV. Length of Toe III surpassing the proximal margin of central subarticular tubercle of Toe IV. Discs of toes I and II unexpanded to moderately expanded, width of toe discs 1.0–1.8 (1.2 ± 0.2) and 1.0–1.8 (1.6 ± 0.2) times the width of their adjacent phalanges, respectively. Discs of toes III, IV, and V moderately to greatly expanded, width of discs, 1.4–2.5 (2.0 ± 0.3), 1.3–2.5 (2.0 ± 0.3) and 1.3–2.0 (1.6 ± 0.2) times the width of their adjacent phalanges, respectively. Paired dorsal digital scutes conspicuous and slightly protuberant in all toes.
Dorsal skin of body shagreened, moderately granular from mid-body to the urostyle region. Forelimb surfaces smooth. Dorsal surface of thighs and shanks with small, scattered tubercles. Ventral skin smooth on throat, chest, belly, and thighs.
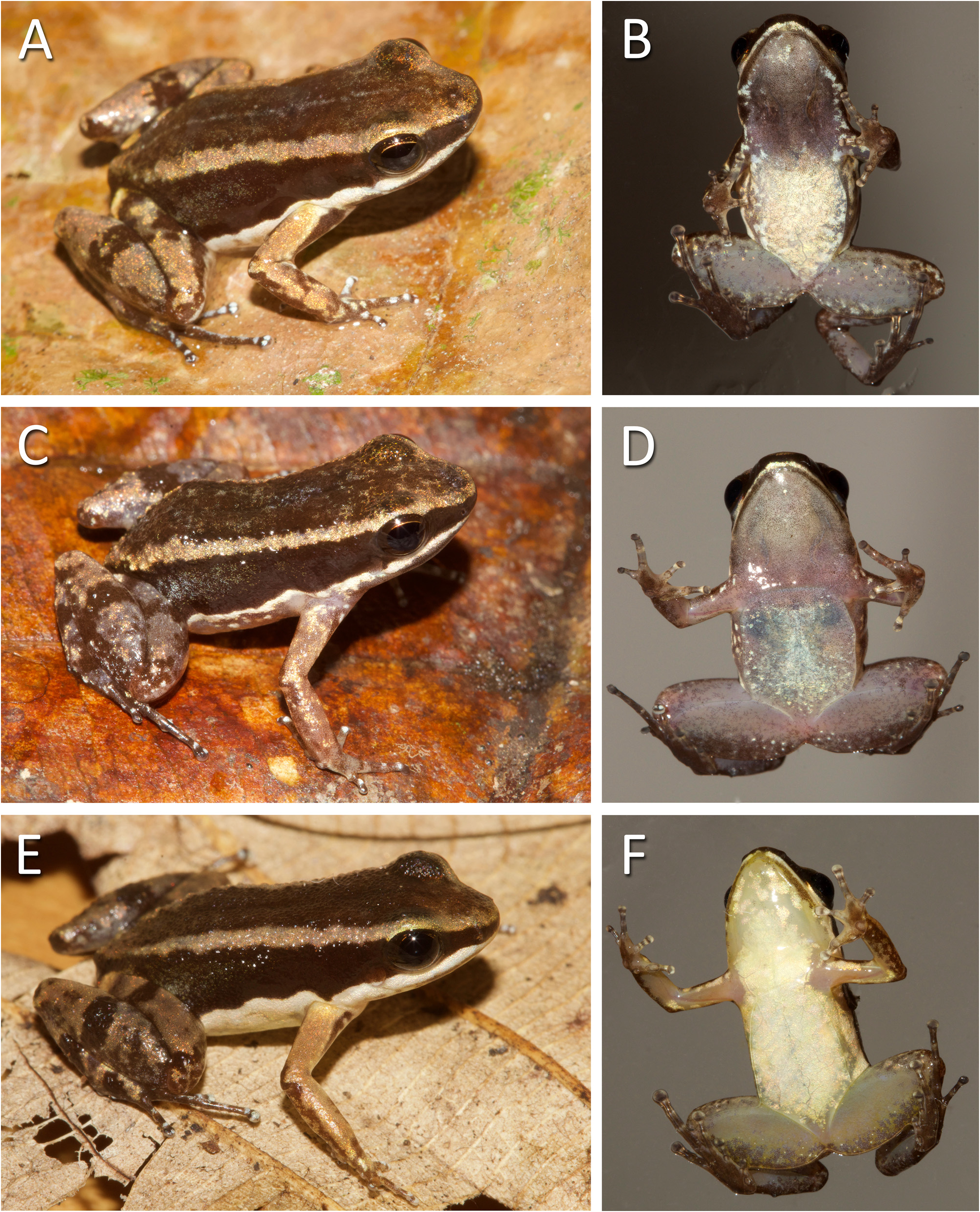
Color in life. We based this description on photographs in life of all collected specimens. Dorsum dark brown, pale tan brown anteriorly, from tip of snout to the level of the eye, and posteriorly, over tip of the urostyle region. A pale, thin vertebral line is present from the level of eye to the urostyle region, in a single specimen ( MCP 14309; Fig. 6A View FIGURE 6 ). Golden, iridescent background color present among dark brown pigments over the orbits. Background color of iris golden, with dark brown reticulation. Pale dorsolateral stripe present, broad, pale tan brown, same color of tips of snout and urostyle region. Lateral surface of body surrounded by a uniformly dark brown stripe from tip of snout to groin, darker than color of dorsum. Dark brown stripe lighter postlaterally, at the inguinal region, where faint spots form a diffuse pale oblique lateral line. Thin, well-defined pale cream oblique lateral line present in some specimens ( Fig. 7A View FIGURE 7 ). Solid, iridescent white ventrolateral stripe present along the lower edge of dark brown stripe from anterior tip of upper lip to groin. Iridescent white blotches or dots present between lower edge of ventrolateral stripe and ventral surface of body, over translucent background, grayish in males, yellowish in females ( Fig. 6 View FIGURE 6 ).
Lower lip dark brown in ventral view. Ventral color of throat, chest and abdomen sexually dimorphic. Throat and vocal sac gray to dark gray in male specimens ( Fig. 6B, D View FIGURE 6 ). Throat and chest translucent pale yellow in female specimens, with iridescent-white patches where underlain by the parietal peritoneum ( Fig. 6F View FIGURE 6 ). Surface of abdomen with scattered white dots or small blotches on translucent gray background in males. Density of white dots extreme- ly variable, rendering brighter or darker abdominal color among male specimens. Surface of abdomen uniformly white to translucent in females, marginally pale yellow.
Dorsal and dorsolateral surfaces of upper arm golden yellow in some individuals, light tan brown or light gray in others ( Fig. 6A, C, E View FIGURE 6 ). Lateral surfaces of upper arm with a longitudinal brown stripe. Dorsal surface of forearm light tan brown, generally with scattered brown blotches; golden blotches present on forearm in some individuals. Iridescent white dots may be present on dorsal and lateral surfaces of upper arm and forearm. Ventral surface of upper arm translucent, peppered with sparse light brown pigments in some individuals. Forearm, carpal and metacarpal regions solid brown ( Fig. 6D, F View FIGURE 6 ). Fingers brown with a varying number of transverse white bars in dorsal view, grayish brown in ventral view. Paired scutes on finger discs iridescent white.
Surfaces immediately adjacent to vent solid dark brown. Paracloacal mark present, short, round or commashaped, light yellow or cream. Paracloacal mark always surrounded by a dark brown frame, continuous with the area adjacent to vent, sometimes continuous with dark brown blotches on dorsal and lateral surface of thigh ( Fig. 6B View FIGURE 6 ). Dorsal surface of thigh uniformly light tan brown. A dark brown blotch may be present medially on dorsal surface of thigh, sometimes extending laterally and forming a transverse dark brown stripe. Additional dark brown blotches may appear proximally on thigh, adjacent to paracloacal mark, or on knee ( Fig. 6A, C, E View FIGURE 6 ). Dorsal surface of shank same color as thigh, with a medial transverse dark brown stripe. Smaller dark brown dots or blotches may be present on dorsal surface of shank. Ventral surface of thigh and shank pale gray to translucent, with a few scattered brown melanophores. White spots and brown blotches present marginally, more densely packed towards the lateral surfaces ( Fig. 6B, D, F View FIGURE 6 ). Tarsal region light tan brown, with a medial dark brown blotch or transverse stripe. Medial transverse stripes on thigh, shank and tarsal region align when posterior limb is in resting position. Ventral surfaces of tarsal and plantar regions brown. Toes brown with a varying number of transverse white bars in dorsal view, brown in ventral view. Paired scutes on toe discs iridescent white, black only on Toe V.
Color pattern of juvenile specimens similar to that of adults ( Fig. 7C, D View FIGURE 7 ). Throat is light gray to translucent, with few scattered light brown pigments. Lower lip weakly pigmented. Upper arm and paracloacal mark pale cream, with no yellow shades.
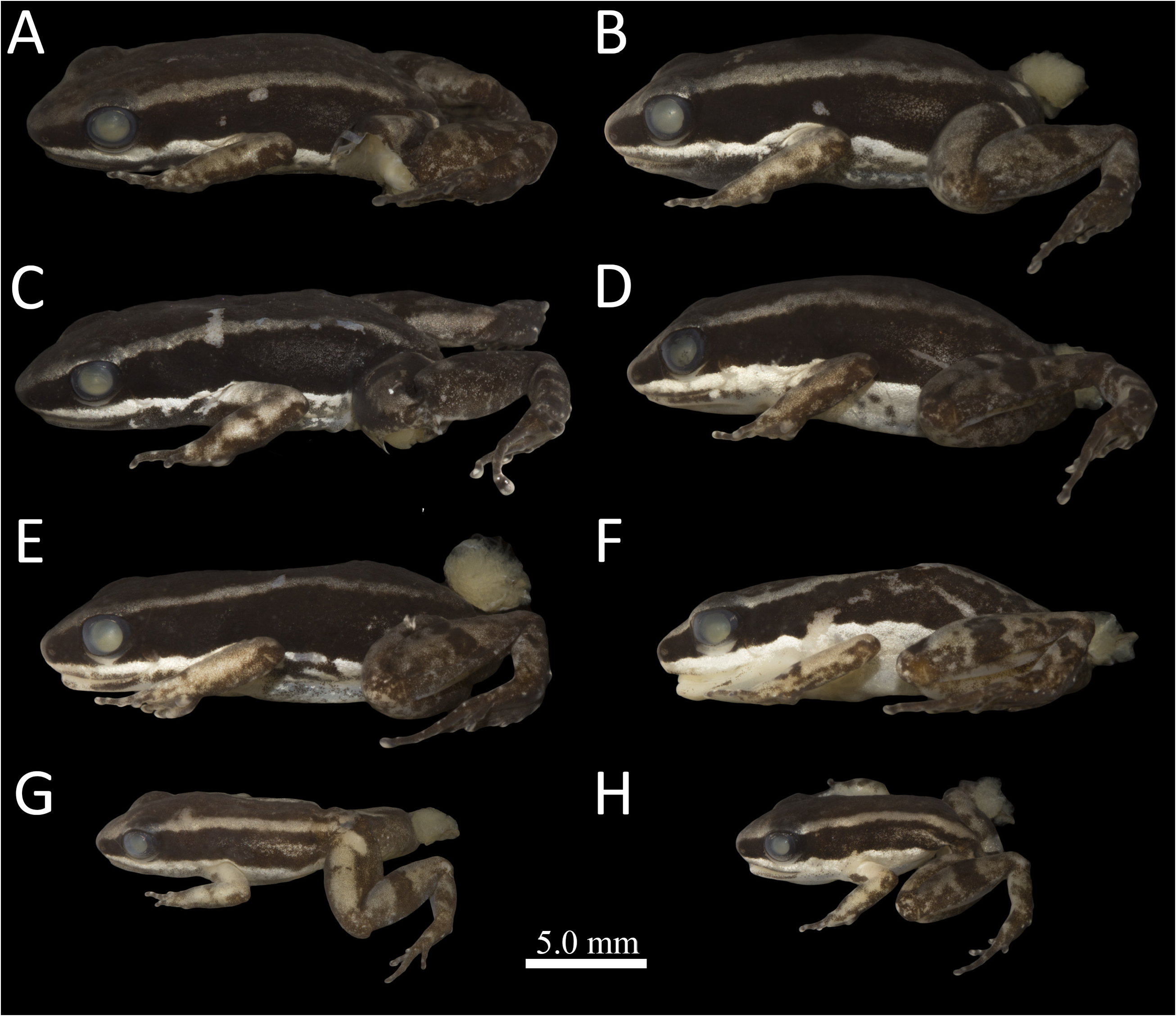
Color in preservative. The description is based on the observation of specimens after one year of preservation. Background color of dorsal surface of body uniform dark brown, lighter on tip of snout and posterior mid body, occasionally with darker irregular spots ( Figs. 8 View FIGURE 8 , 9 View FIGURE 9 ). Color of dorsum darker over eyelid. A thin vertebral line can be present, extending from the interorbital region to urostyle. Pale dorsolateral stripe present, solid, straight, extending from tip of snout to the urostyle region, merging with lighter color of the urostyle region in some specimens. Inner and outer edges of dorsolateral stripe well-defined ( Figs. 8 View FIGURE 8 , 9 View FIGURE 9 ). Lateral surface of body with a solid dark brown stripe, extending from tip of snout to groin ( Fig. 10 View FIGURE 10 ). Diffuse light brown stripe present on lateral solid dark brown stripe at inguinal region, extending from groin to midbody, without reaching the lower or upper edges of lateral dark brown stripe. Solid, iridescent white ventrolateral stripe present along the lower edge of lateral dark brown stripe, extending from anterior tip of upper lips, crossing above the insertion of the forelimb and ending on groin ( Fig. 10 View FIGURE 10 ). Dark brown mottling present below white ventrolateral stripe, extending from the upper and lower lips to groin, reaching the ventral surface. Ventrolateral dark mottling denser in males. Iridescent white irregular spots present among ventrolateral brown mottling, more frequent towards the belly ( Fig. 10A–C View FIGURE 10 ).
Background color of ventral surfaces cream to translucent ( Figs. 11 View FIGURE 11 , 12 View FIGURE 12 ). Color of throat, chest and abdomen sexually dimorphic. Throat and chest densely covered with dark gray melanophores in males, density of dark melanophores variable among specimens ( Fig. 11 View FIGURE 11 ). Throat and chest of females light cream, with white iridescent patches, more densely packed on chest than on throat ( Fig. 12 View FIGURE 12 ). Dark gray mottling present on lower lip and anterior portion of throat in females ( Fig. 12A–D View FIGURE 12 ). Belly surface with pale gray melanophores in males, peppered with iridescent white spots. White iridescent spots more densely concentrated marginally, towards lateral surface of abdomen, producing iridescent white mottling ( Fig. 11 View FIGURE 11 ). Belly iridescent white in females, with light brownish-gray mottling in some specimens ( Fig. 12 A–D View FIGURE 12 ).
Dorsal surface on forelimbs cream to light brown, with dark brown patches on elbow, forearm and wrist ( Figs. 8 View FIGURE 8 , 9 View FIGURE 9 ). Fingers III and IV more darkly pigmented than fingers I and II. Tips of all fingers with a white, solid, iridescent spot in dorsal view, approximately at the base of finger discs. Paired scutes on discs of fingers I, II and III conspicuous, iridescent white, faded white on finger IV. Upper arm sexually dimorphic in ventral view. Brown mottled with iridescent irregular spots in males ( Fig. 11 View FIGURE 11 ). Light cream in females ( Fig. 12 A–D View FIGURE 12 ). Outer surface of forearm, carpal and metarcarpal regions solid brown, densely pigmented.
Area immediately around vent dark brown. White paracloacal mark conspicuous, round or comma-shaped, broader at its proximal end, bordered dark brown ( Figs. 8 View FIGURE 8 , 9 View FIGURE 9 ). Dorsal surface of thigh light brown. An irregular transversal dark brown blotch or bar is present medially on dorsal surface of thigh in 70% of the specimens. Smaller, additional dark brown blotches, irregular in shape, present on distal and proximal dorsal surfaces of thigh in some specimens, sometimes merging with solid dark brown color of inner and outer dorsolateral surface of thigh. Dorsal surface of shank same color as thigh, with a single dark brown blotch present medially and smaller, irregular dark brown spots. Dorsal surface of tarsal region same color as thigh and shank, with patterns of transversal dark brown blotches or bars, varying from uniformly cream ( Fig. 8E View FIGURE 8 ) to a fully striped pattern ( Fig. 8D View FIGURE 8 ). Dorsal surface of toes dark brown, more densely pigmented on toes III, IV and V. Paired scutes white on toes I, II, III and IV, black on Toe V. Ventral surfaces of thigh and shank cream, with dark brown mottling on marginal edges and white scattered spots on outer edge. Ventral surfaces of tarsal and metatarsal regions uniformly dark brown. Toes dark brown, densely pigmented in ventral view.
Juveniles with the same color pattern as adults, but lighter in dorsal view ( Fig. 9E, F View FIGURE 9 ). Ventrally, the color pattern is similar to that of adult females ( Fig. 12 View FIGURE 12 ). Throat, chest, and belly cream with scattered dark gray mottling on throat. Ventral surfaces iridescent white ( Fig. 12E, F View FIGURE 12 ).
Bioacoustics. During fieldwork, we only heard and recorded a single type of vocalization, which we consider the advertisement call of Allobates trilineatus . We recorded the vocalizations of three specimens, which were all collected.
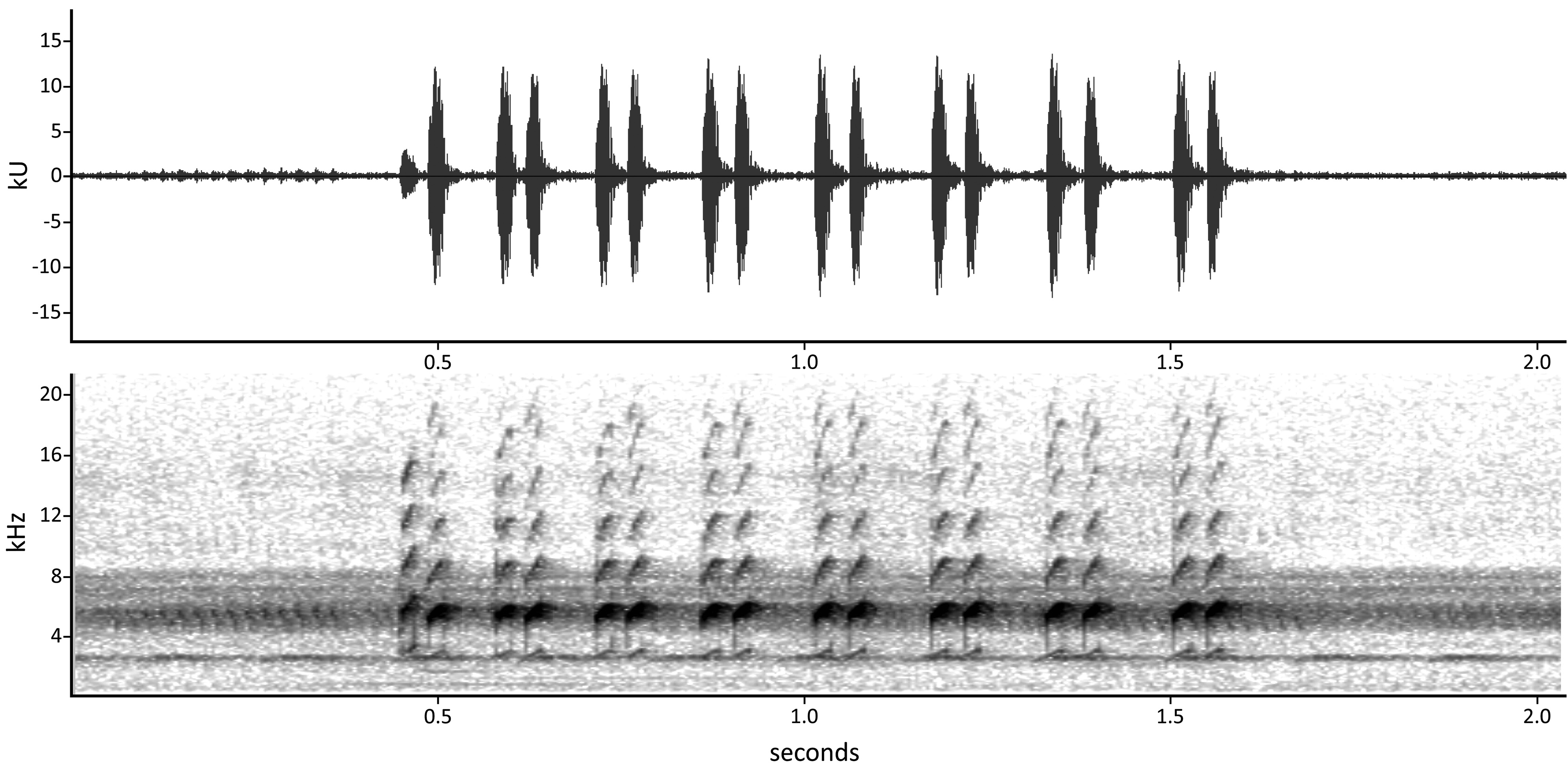
The advertisement call of Allobates trilineatus is emitted as discrete groups of note-pairs ( Fig. 13 View FIGURE 13 , Table 2 View TABLE 2 ). The number of note-pairs in each group varies between 6–11 note-pairs/group (10 ± 1). Duration of note-pair groups varies between 0.96–1.61 (1.24 ± 0.17) s. Within each group, note-pairs are emitted at a rate of 5.2–8.7 (6.8 ± 0.82) note-pairs/s. Note-pair groups are emitted between relatively long silent intervals, with duration of silent intervals between consecutive note-pair groups ranging between 7.04–46.65 (13.80 ± 8.90) s. Duration of note-pairs varies between 0.06–0.08 (0.07 ± 0.004) s. Duration of individual notes within a note-pair range between 0.02–0.03 (0.02 ± 0.001) s. Notes forming a note pair are split by a very short silent interval of 0.01–0.03 (0.02 ± 0.002) s. Duration of silent intervals between consecutive note-pairs ranges between 0.06–0.16 (0.10 ± 0.03) s. On average, there is no difference between the first and second notes of a note pair considering their duration and spectral parameters ( ANOVA: F 1,59 <1.7, P > 0.2 in all tests). However, the first note at the onset of each note-pair group is emitted with a lower amplitude and occupies a narrower frequency bandwidth than the following notes. Peak frequency of notes varies between 5.06–5.81 (5.33 ± 0.22) kHz. Lower frequency of notes ranges between 4.67–5.34 (4.98 ± 0.20) kHz and the upper frequency between 5.25–6.15 (5.63 ± 0.24) kHz. Bandwidth of notes range between 0.34–0.95 (0.65 ± 0.15) kHz. In recordings with very low background noise, the fundamental frequency of notes is visible at approximately 2.70 kHz, and up to six harmonics are detected, the second corresponding to the dominant frequency.
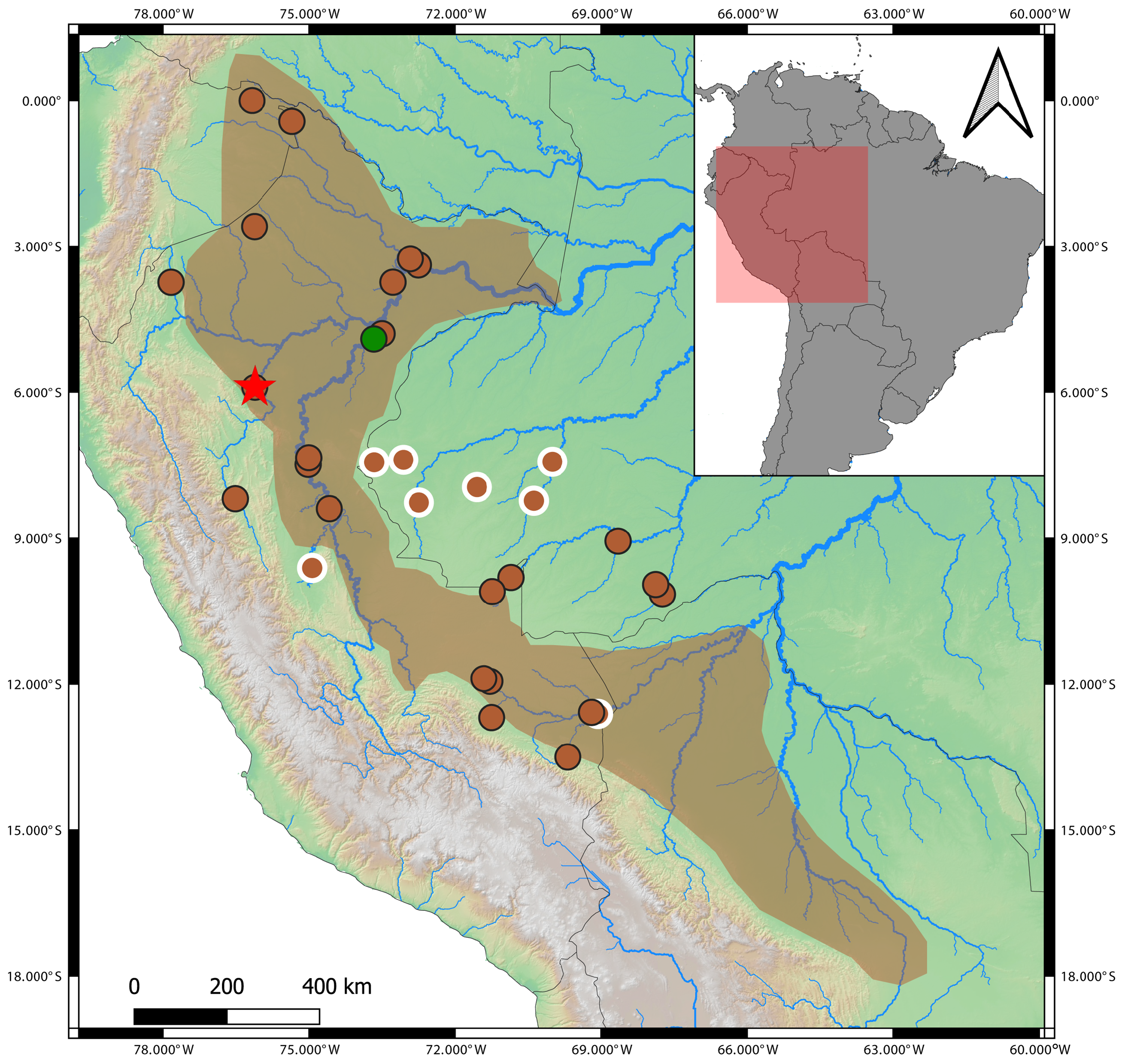
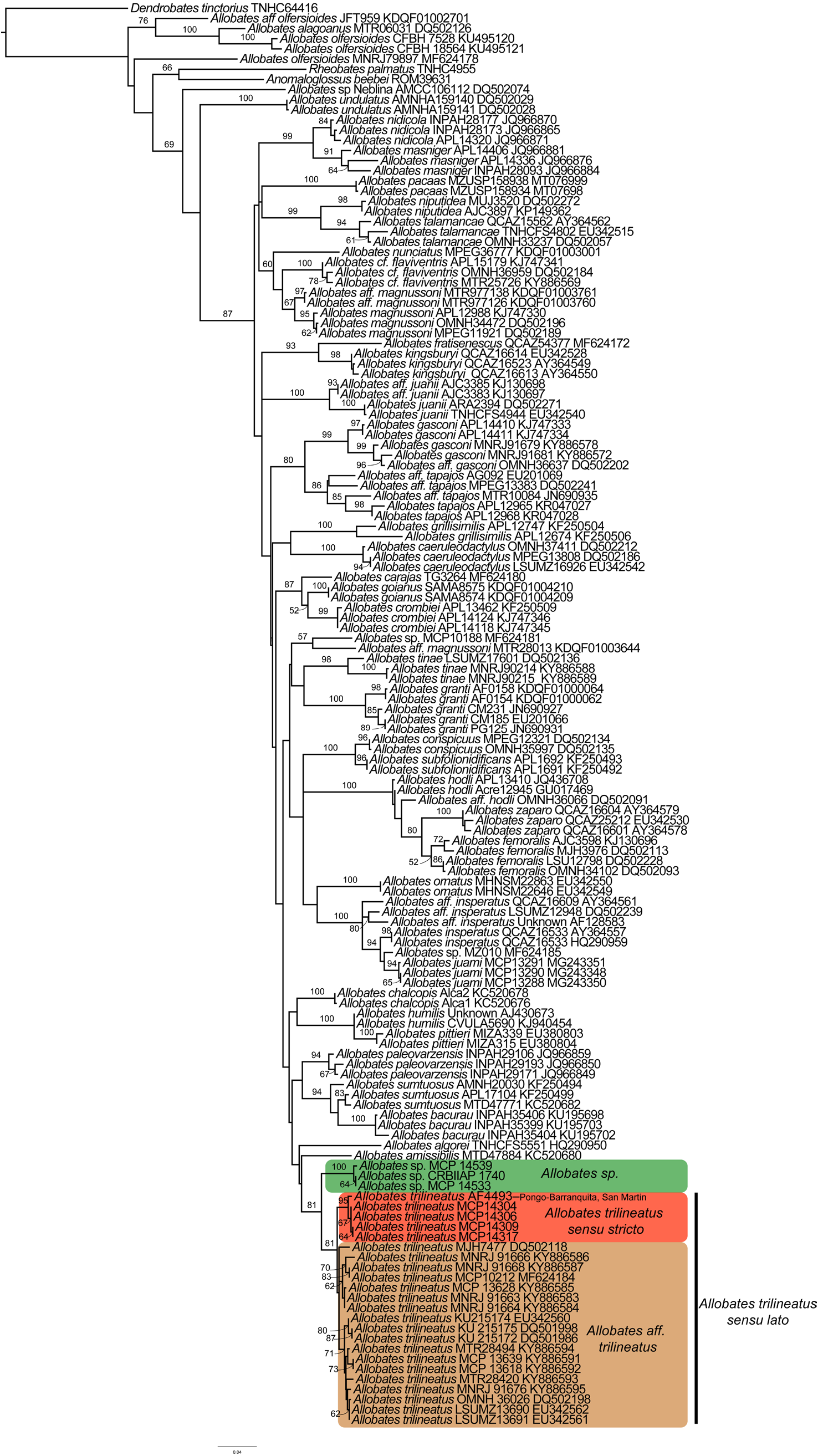
Phylogenetics. Our similarity alignment of the 16S rRNA mitochondrial gene contained 605 bp and 140 terminals. The best-fit evolutionary model (log likelihood = -8568.57, AICc = 18232.63) was GTR+I+G. The most optimal tree was well resolved, with 9% polytomies among 139 possible clades. Polytomies involve shallow clades of two or three species ( Fig. 14 View FIGURE 14 ). In this phylogenetic tree, Allobates is paraphyletic regarding Anomaloglossus beebei and Rheobates palmatus , but relationships among different genera had very weak bootstrap support ( Fig. 14 View FIGURE 14 ). The topotypic samples of Allobates trilineatus are monophyletic and sister to a sample ( AF 4493) of A. trilineatus from Pongo-Barranquita, department of San Martin, Peru ( Fig. 14 View FIGURE 14 ). We hereafter refer to this clade as Allobates trilineatus sensu stricto ( s.s.). This clade is sister to a clade formed by all published sequences of A. trilineatus by Grant et al. (2006) and Melo-Sampaio et al. (2018) from Acre, Brazil, and from southern Peru ( Figs. 2 View FIGURE 2 , 14 View FIGURE 14 ). This latter clade is hereafter referred to as A. aff. trilineatus . Consequently, we refer to the clade formed by A. aff. trilineatus and A. trilineatus s.s. as A. trilineatus sensu lato ( s.l.).
Samples of Allobates from Jenaro-Herrera, in Loreto, Peru, form a clade sister to A. trilineatus s.l. The clade formed by A. trilineatus s.l. and the Allobates species from Jenaro-Herrera is the sister clade to Allobates amissibilis .
Uncorrected-pairwise genetic distances within A. trilineatus s.s. is 0.0–0.9%. We provide a summary of the genetic distances within the A. trilineatus s.l. and the closely related clade represented by specimens from Jenaro- Herrera in Table 3 View Table 3 . Genetic distances of samples in the A. trilineatus s.l. clade and the remaining congeneric species included in the phylogenetic analysis were always higher than 6.0%.
Natural history. In the village of Munichis, Yurimaguas, we found Allobates trilineatus in a patch of secondary-growth terra firme rainforest, where it was locally common. Specimens were active during the day, males calling more actively near the sunset, between 17:00 and 18:30 h. We found all specimens moving or calling on the leaf litter on the forest floor. Syntopic diurnal species included Adenomera sp., Amazophrynella sp., Ameerega hahneli , and Rhinella aff. margaritifera . Eggs, tadpoles, and detailed information on the reproductive behavior of A. trilineatus remain unknown.
Diagnosis. Morphological diagnosis. Based on our new species definition, we compared Allobates trilineatus s.s. with all Allobates species from Amazonia. Character states of A. trilineatus are in parentheses. Preserved males of A. amissibilis Kok, Hölting, & Ernst, 2013 , A. brunneus (Cope, 1887) , A. caeruleodactylus ( Lima & Caldwell, 2001), A. carajas Simões, Rojas , & Lima, 2019, A. crombiei ( Morales, 2002) , A. flaviventris Melo-Sampaio, Souza, & Peloso, 2013 , A. granti (Kok, MacCulloch, Gaucher, Poelman, Bourne, Lathrop, & Lenglet, 2006) , A. grillisimilis Simões, Sturaro, Peloso , & Lima, 2013, A. insperatus ( Morales, 2002) , A. juami Simões, Gagliardi-Urrutia, Rojas-Runjaic, & Castroviejo-Fisher, 2018 , A. juanii ( Morales, 1994) , A. magnussoni Lima, Simões, & Kaefer, 2014, A. mcdiarmidi (Reynolds & Foster, 1992) , A. ornatus ( Morales, 2002) , A. subfolionidificans ( Lima, Sanchez, & Souza, 2007), A. sumtuosus ( Morales, 2002) , A. tapajos Lima, Simões, & Kaefer, 2015, and A. tinae Melo-Sampaio, Oliveira, & Prates, 2018 have throat, chest and belly cream with scattered melanophores mainly on chin and occasionally extending from throat to belly; vocal sacs generally cream (males with dark gray throat, chest, and belly; vocal sac dark gray in live and preserved specimens). In A. brunneus , A. carajas , A, crombiei , A. flaviventris , A. juanii , A. magnussoni , A. ornatus , and A. tapajos , the dorsolateral stripe broadens posteriorly to eyelid forming an hourglass or wavy pattern (pale dorsolateral stripe solid and straight, not broadening on any region posterior to the eye). Pale dorsolateral stripe absent in A. amissibilis , A. caeruleodactylus , A. granti , A. subfolionidificans (pale dorsolateral stripe present and conspicuous). Finger III not swollen in males of A. grillisimilis , A. insperatus , A. juami , A. sumtuosus , A. tapajos , and A. tinae (preaxial side of Finger III swollen).
Live specimens of Allobates femoralis ( Boulenger, 1884) , A. hodli Simões , Lima, & Farias, 2010, A. myersi (Pyburn, 1981) , and A. zaparo (Silverstone, 1976) have bright yellow, orange or red flash marks on dorsal surface of thigh in life, and black and white marbling on belly and ventral surface of thigh in preserved and live specimens (yellow, orange or red flash marks on thigh and black and white marbling absent). Adults of these species are also larger, with SVL = 22.2–28.1 mm in A. hodli , 26.4–32.8 mm in A. myersi , and 16.5–31.5 mm in A. zaparo (SVL = 14.6–16.6 mm).
Allobates marchesianus (Melin, 1941) has granular dorsal skin, inconspicuous pale ventrolateral stripe, Finger III not swollen in adult males, and tip of Toe III reaching the proximal margin of the central subarticular tubercle of Toe IV (skin of dorsum shagreened, conspicuous white ventrolateral stripe, swollen Finger III in adult males, and tip of Toe III surpassing the central subarticular tubercle of Toe IV).
Allobates masniger ( Morales, 2002) , A. nidicola (Caldwell & Lima, 2003), and A. nunciatus Moraes, Pavan , & Lima, 2019 lack pale dorsolateral stripe (pale dorsolateral stripe present and conspicuous), Finger III not swollen in males (Finger III swollen on preaxial side), tip of Finger III reaching the proximal margin of the central subarticular tubercle of Toe IV in A. nidicola (Toe III surpassing the central subarticular tubercle of Toe IV). Larger adults, with SVL = 17.9–20.1 mm in A. masniger , 18.5–21.4 mm in A. nidicola , 21.1–23.4 mm in A. nunciatus ( 14.6–16.6 mm).
Allobates melanolaemus ( Grant & Rodriguez, 2001) and A. paleovarzensis Lima, Caldwell, Biavati, & Montanarin, 2010 have granular skin on dorsal surfaces (skin on dorsum shagreened), a pale dorsolateral and ventrolateral stripes weakly marked, sometimes diffuse and inconspicuous, in A. paleovarzensis (dorsolateral and ventrolateral stripes solid and conspicuous), Finger III hardly swollen in adult males of A. paleovarzensis and not swollen in adult males of A. melanolaemus (Finger III markedly swollen in adult males), larger adults, with SVL = 21.1–23.4 mm in A. melanolemus and 18.3–22.4 mm in A. paleovarzensis ( 14.6–16.6 mm).
Allobates pacaas Melo-Sampaio, Prates, Peloso, Recoder, Dal Vechio, Marques-Souza & Rodrigues, 2020 has a dorsum with hourglass-shaped pattern and adult males with Finger III not swollen on preaxial side (dorsal coloration uniform, with no hourglass pattern, Finger III of adult males swollen on preaxial side). Allobates bacurau Simões, 2016 has a dorsum moderately granular, tan brown, and ventral surfaces with no iridescent white marks or spots in live and preserved specimens (dorsum shagreened, darker brown, iridescent marks or spots present on ventral surfaces).
Acoustic diagnosis. The advertisement call formed by the emission of groups of note-pairs distinguishes Allobates trilineatus s.s. from all congeneric species, except A. granti , A. hodli , A. kingsburyi , A. magnussoni and A. tapajos . Note-pairs are emitted continuously, not arranged in discrete groups, in advertisement calls of A. magnussoni , and A. tapajos . The first note of each note-pair in calls of A. magnussoni is approximately twice as long as the second note ( Lima et al. 2014) and the first and second notes are also split by a 0.07– 0.12 s silent interval, while both notes are of similar duration and longer than silent interval between notes in A. trilineatus s. s. In calls of A. tapajos , notes within the same note-pair are split by a 0.11– 0.16 s silent interval, and consecutive note pairs are separated by a 0.30– 0.49 s silent interval ( Lima et al. 2015), much longer than silent intervals between notes and note-pairs of A. trilineatus s.s.
Notes of calls of Allobates flaviventris are emitted with a peak frequency of 4.3–4.6 kHz, lower than peak frequency of notes of A. trilineatus s.s., and consecutive note-pairs are split by a longer silent interval, ranging between 0.31– 0.41 s ( Lima et al. 2014). Note-pair groups are narrowly spaced in A. granti , duration of silent interval between consecutive groups ranging between 0.46– 1.78 s ( Kok et al. 2006; Kok & Ernst 2007), much shorter than in A. trilineatus s.s. Calls of A. hodli are emitted with a peak frequency ranging between 2.9–3.6 kHz, much lower than peak frequency of calls of A. trilineatus s.s.; duration of note-pairs in calls of A. hodli are also longer, ranging between 0.14– 0.20 s ( Simões et al. 2010). Groups of note-pairs are longer, ranging between 16.30– 25.43 s and containing 34–96 note-pairs in A. kingsburyi ; notes are also emitted with a peak frequency of 3.0–4.7 kHz (Castillo- Trenn & Coloma 2008), lower than that of A. trilineatus .
.
Geographic distribution. The type series of Allobates trilineatus was described from Yurimaguas, Alto Amazonas, Loreto, Peru. We collected our new samples from Munichis village, Yurimaguas, approximately 12.5 km west of Yurimaguas airport. A single sample (AF4493) collected from Pongo-Barranquita, department of San Martin, Peru, about 44 km southwest of Yurimaguas, is closely related to topotypic samples ( Fig. 14 View FIGURE 14 ). These localities are situated in the interfluve between the rivers Marañon and Huallaga ( Fig. 2 View FIGURE 2 ) and it is reasonable to suggest that A. trilineatus s.s. is distributed in more localities within this interfluve. Based on molecular evidence ( i.e., uncorrectedpairwise genetic distances <3% and sister phylogenetic relationship), specimens from the Brazilian state of Acre and from southern Peru in Panguana, Huanuco, and Puerto Maldonado, Madre de Dios, are herein identified as A. aff. trilineatus , pending examination of morphological and acoustic diagnostic characters. Additionally, we suggest that earlier records of A. trilineatus from Amazonian localities of Colombia, Ecuador, and northern Peru ( Fig. 2 View FIGURE 2 ) should be checked for consistency with our phenotypic and molecular data.
Table 3. Uncorrected-pairwise genetic distances (in percentage) among specimens of Allobates trilineatus species complex based on a similarity alignment of 563 bp fragment of 16S without missing data. 1–3: Sequences from specimens collected in Jenaro-Herrera, Loreto, Peru, which correspond to a new candidate species. 4–21: Sequences from specimens collected in several localities in northwestern Brazil and southern Peru. 22–26: Sequences from topotypic specimens (Yurimaguas, Loreto) and Pongo-Barranquita, San Martin, both in Peru.
| Taxa | Voucher | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 CRBIIAP 1740 | 0.0 | ||||||||||||||||||||||||||
| Allobates sp. | 2 MCP 14533 | 0.0 | 0.0 | ||||||||||||||||||||||||
| 3 MCP 14539 | 0.5 | 0.5 | 0.0 | ||||||||||||||||||||||||
| 4 MJH 7477 | 4.7 | 4.7 | 4.8 | 0.0 | |||||||||||||||||||||||
| 5 KU 215175 | 4.8 | 4.8 | 4.8 | 2.3 | 0.0 | ||||||||||||||||||||||
| 6 KU 215172 | 4.8 | 4.8 | 4.8 | 2.3 | 0.0 | 0.0 | |||||||||||||||||||||
| 7 KU215174 | 4.5 | 4.5 | 4.5 | 2.0 | 0.4 | 0.4 | 0.0 | ||||||||||||||||||||
| 8 MTR28420 | 4.5 | 4.5 | 4.5 | 1.6 | 1.3 | 1.3 | 0.9 | 0.0 | |||||||||||||||||||
| 9 MNRJ 91676 | 4.8 | 4.8 | 4.8 | 2.0 | 1.2 | 1.2 | 0.9 | 0.7 | 0.0 | ||||||||||||||||||
| 10 OMNH 36026 | 4.8 | 4.8 | 4.8 | 2.0 | 1.2 | 1.2 | 0.9 | 0.7 | 0.7 | 0.0 | |||||||||||||||||
| 11 LSUMZ13690 | 4.7 | 4.7 | 4.7 | 1.8 | 1.1 | 1.1 | 0.7 | 0.5 | 0.5 | 0.2 | 0.0 | ||||||||||||||||
| Allobates aff. trilineatus | 12 LSUMZ13691 13 MCP 13639 | 4.7 4.3 | 4.7 4.3 | 4.7 4.3 | 1.8 1.8 | 1.1 1.4 | 1.1 1.4 | 0.7 1.1 | 0.5 0.5 | 0.5 1.1 | 0.2 1.1 | 0.0 0.9 | 0.0 0.9 | 0.0 | |||||||||||||
| 14 MCP 13618 | 4.3 | 4.3 | 4.3 | 1.8 | 1.4 | 1.4 | 1.1 | 0.5 | 1.1 | 1.1 | 0.9 | 0.9 | 0.0 | 0.0 | |||||||||||||
| 15 MTR28494 | 4.5 | 4.5 | 4.5 | 1.6 | 1.2 | 1.2 | 0.9 | 0.9 | 0.9 | 0.9 | 0.7 | 0.7 | 0.7 | 0.7 | 0.0 | ||||||||||||
| 16 MNRJ 91666 | 5.5 | 5.5 | 5.7 | 2.1 | 2.9 | 2.9 | 2.5 | 2.5 | 2.5 | 2.5 | 2.3 | 2.3 | 2.7 | 2.7 | 2.1 | 0.0 | |||||||||||
| 17 MCP10212 | 4.7 | 4.7 | 4.8 | 1.8 | 2.3 | 2.3 | 2.0 | 2.0 | 2.0 | 2.0 | 1.8 | 1.8 | 2.0 | 2.0 | 1.4 | 1.2 | 0.0 | ||||||||||
| 18 MNRJ 91668 | 4.7 | 4.7 | 4.8 | 1.8 | 2.3 | 2.3 | 2.0 | 2.0 | 2.0 | 2.0 | 1.8 | 1.8 | 2.0 | 2.0 | 1.4 | 1.2 | 0.0 | 0.0 | |||||||||
| 19 MNRJ 91664 | 4.5 | 4.5 | 4.7 | 1.6 | 1.8 | 1.8 | 1.4 | 1.8 | 1.8 | 1.8 | 1.6 | 1.6 | 2.0 | 2.0 | 1.4 | 1.4 | 0.9 | 0.9 | 0.0 | ||||||||
| 20 MNRJ 91663 | 4.5 | 4.5 | 4.7 | 1.6 | 1.8 | 1.8 | 1.4 | 1.8 | 1.8 | 1.8 | 1.6 | 1.6 | 2.0 | 2.0 | 1.4 | 1.4 | 0.9 | 0.9 | 0.0 | 0.0 | |||||||
| 21 MCP 13628 | 4.7 | 4.7 | 4.8 | 1.8 | 2.0 | 2.0 | 1.6 | 2.0 | 2.0 | 2.0 | 1.8 | 1.8 | 2.1 | 2.1 | 1.6 | 1.4 | 1.1 | 1.1 | 0.2 | 0.2 | 0.0 | ||||||
| 22 AF4493 | 5.7 | 5.7 | 5.9 | 2.8 | 3.1 | 3.1 | 2.8 | 3.1 | 3.5 | 3.1 | 3.1 | 3.1 | 2.8 | 2.8 | 3.1 | 3.8 | 2.8 | 2.8 | 2.1 | 2.1 | 2.4 | 0.0 | |||||
| 23 MCP 14309 | 5.0 | 5.0 | 5.2 | 2.7 | 2.9 | 2.9 | 2.5 | 2.9 | 2.9 | 2.7 | 2.5 | 2.5 | 2.5 | 2.5 | 2.3 | 3.1 | 2.2 | 2.2 | 1.8 | 1.8 | 2.0 | 0.9 | 0.0 | ||||
| Allobates trilineatus s.s. | 24 MCP 14317 | 5.0 | 5.0 | 5.2 | 2.7 | 2.9 | 2.9 | 2.5 | 2.9 | 2.9 | 2.7 | 2.5 | 2.5 | 2.5 | 2.5 | 2.3 | 3.1 | 2.2 | 2.2 | 1.8 | 1.8 | 2.0 | 0.9 | 0.0 | 0.0 | ||
| 25 MCP 14304 | 4.9 | 4.9 | 5.1 | 2.5 | 2.7 | 2.7 | 2.3 | 2.7 | 2.7 | 2.5 | 2.3 | 2.3 | 2.3 | 2.3 | 2.2 | 2.9 | 2.0 | 2.0 | 1.6 | 1.6 | 1.8 | 0.7 | 0.2 | 0.2 | 0.0 | ||
| 26 MCP 14306 | 4.9 | 4.9 | 5.0 | 2.5 | 2.7 | 2.7 | 2.3 | 2.7 | 2.7 | 2.5 | 2.3 | 2.3 | 2.3 | 2.3 | 2.2 | 2.9 | 2.0 | 2.0 | 1.6 | 1.6 | 1.8 | 0.7 | 0.2 | 0.2 | 0.0 | 0.0 |
Table 3. Uncorrected-pairwise genetic distances (in percentage) among specimens of Allobates trilineatus species complex based on a similarity alignment of 563 bp fragment of 16S without missing data. 1–3: Sequences from specimens collected in Jenaro-Herrera, Loreto, Peru, which correspond to a new candidate species. 4–21: Sequences from specimens collected in several localities in northwestern Brazil and southern Peru. 22–26: Sequences from topotypic specimens (Yurimaguas, Loreto) and Pongo-Barranquita, San Martin, both in Peru.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.