Carmenta chromolaenae Eichlin
|
publication ID |
https://doi.org/ 10.5281/zenodo.191323 |
|
DOI |
https://doi.org/10.5281/zenodo.6214344 |
|
persistent identifier |
https://treatment.plazi.org/id/03D887B5-FFE4-BC34-BEFA-833E33DEFB9E |
|
treatment provided by |
Plazi |
|
scientific name |
Carmenta chromolaenae Eichlin |
| status |
sp. nov. |
Carmenta chromolaenae Eichlin View in CoL , new sp.
Figs. 1–10 View FIGURES 1 – 4 View FIGURES 5 – 6 View FIGURES 7 – 8 View FIGURES 9 – 10
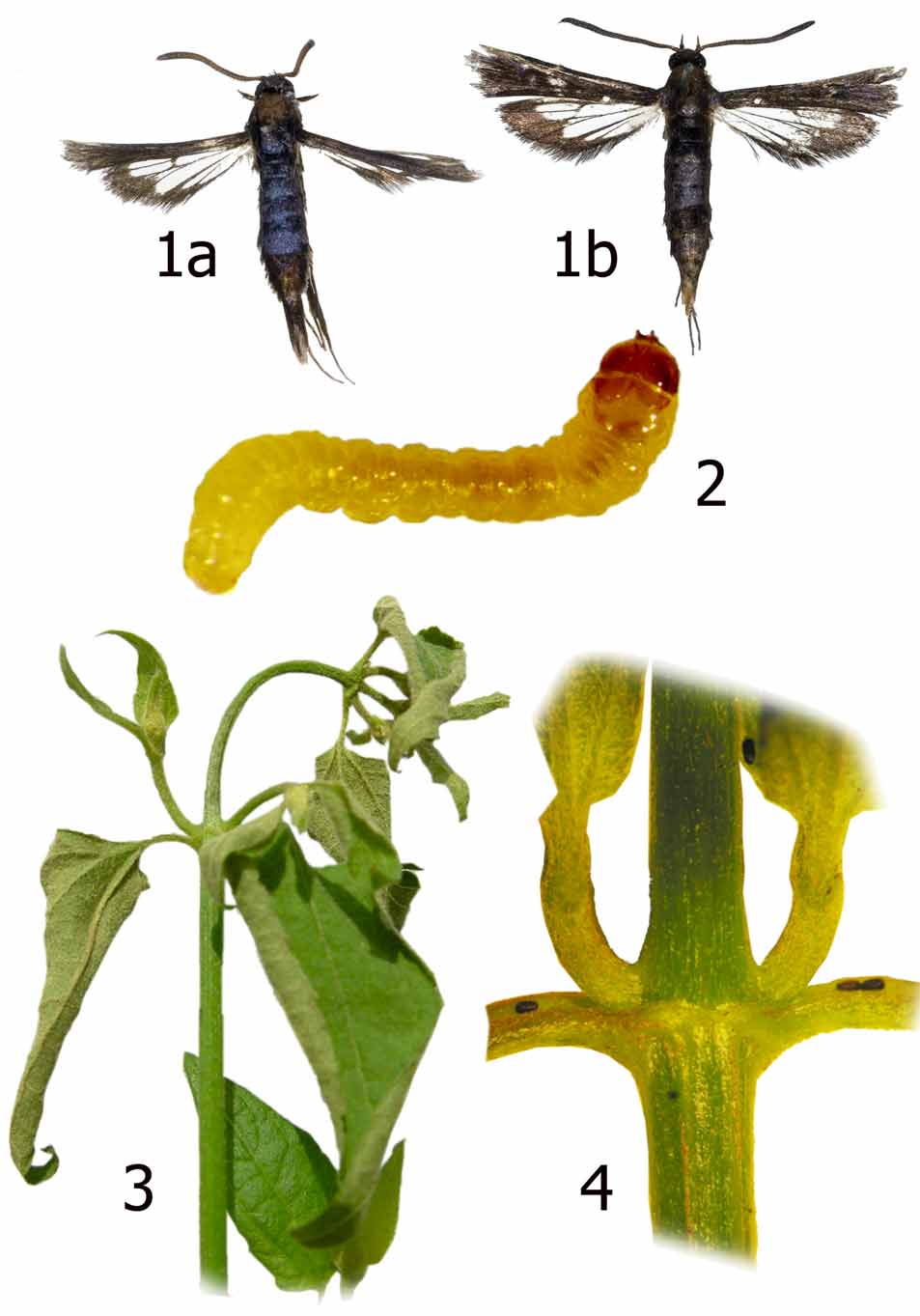
Diagnosis. Carmenta chromolaenae is a small (forewing length 4–7 mm), darkly colored moth with dark opaque forewings, and hind wings that are about two-thirds clear but with the outer one-third dark opaque. It resembles C. unicolor (Walker) from Bolivia and Brazil. The latter species is somewhat larger (forewing length 8–10 mm) and differs by having the hind wing more opaque, and on the head the occipital fringe has some pale yellow or white and some white on the front laterally.
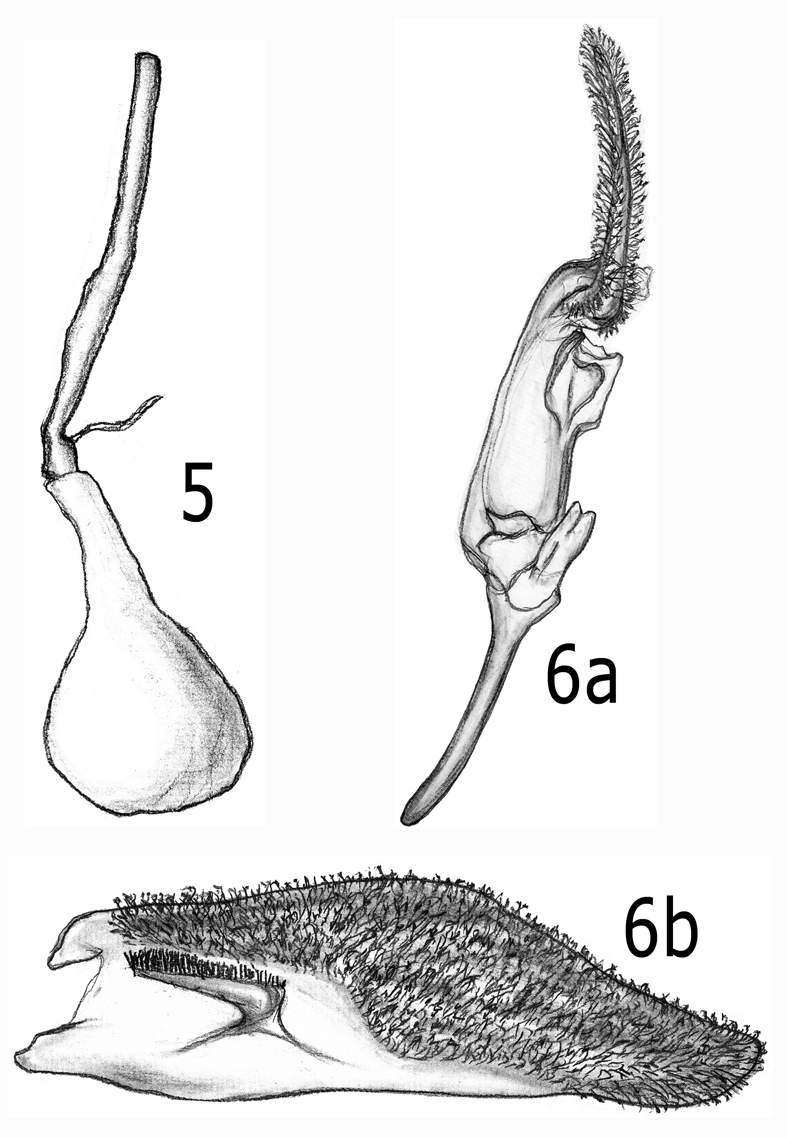
Description. Male ( Fig. 1 View FIGURES 1 – 4 ). Head: Vertex blue black; front shiny blue black; occipital fringe blue black; labial palpus white basally and ventrally, blue black dorsally and apically; antenna blue black. Thorax: Blue black dorsally; brown black laterally and ventrally. Legs mostly brown black; forecoxa mostly white; tarsi white ventrally and around tarsal joints, some pale yellow to white powdered dorsally on hind tibiae. Fore wing dorsally entirely opaque, blue black; ventrally brown black but pale yellow to white on posterior margin from edge of discal cell distally to vein CuA; white scaling between veins on distal area (often less so on some females). Hind wing mostly hyaline but with broad brown black distal margin, extending proximally to include all of cell between M3 and CuA1 and most of cell between CuA1 and CuA2; ventrally with white at wing base and pale yellow on anal margin. Abdomen: Dorsally blue black, without pale banding; ventrally pale yellow to white. Male genitalia ( Fig. 6 View FIGURES 5 – 6 ) with valva elongate, bluntly pointed distally; atypical for Carmenta spp., crista sacculi short, centered basally, not approaching ventral margin of valva; saccus narrow, about onehalf length of valva; scopula androconialis elongate, about half length of valvae.
Female. Head and thorax: Essentially as described for male, except antenna without ventral cilia; hind wing opaque region perhaps slightly broader; occasionally with less pale scaling ventrally on the abdomen and ventrally on the wings. Abdomen: Genitalia ( Fig. 5 View FIGURES 5 – 6 ) with antrum slender, elongate, somewhat sclerotized, slightly pigmented, mostly straight, slightly restricted near ductus bursae; ductus bursae broader than antrum, expanding gradually to ovoid corpus bursae, without stigma.
Eggs ( Fig. 4 View FIGURES 1 – 4 ): Brown, oval, dorsoventrally flattened with concave upper surface, thick chorion with longitudinal ridges, particularly at one end.
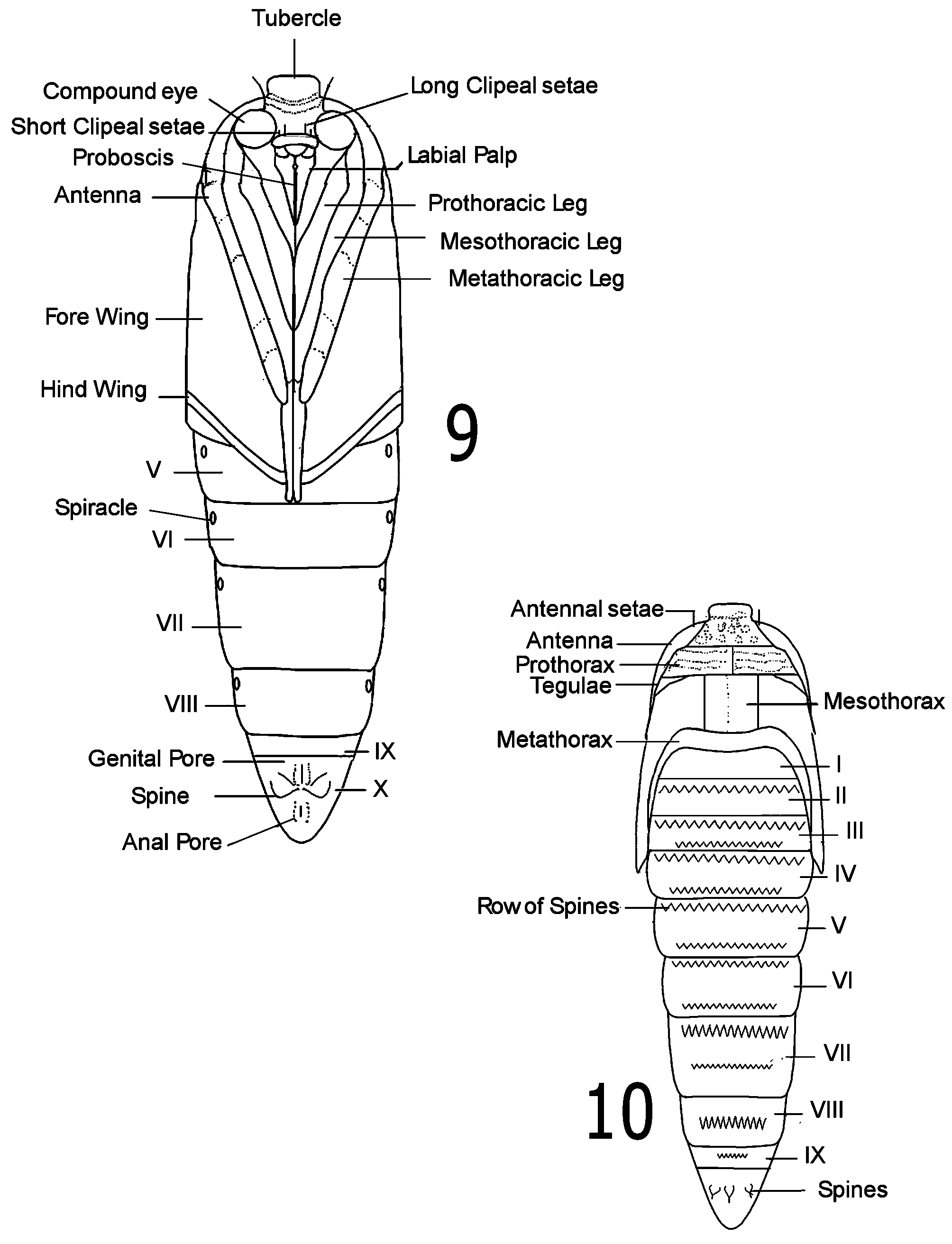
Larva ( Figs. 2 View FIGURES 1 – 4 , 7, 8 View FIGURES 7 – 8 ). General: Spiracles oval, simple on A3 to A8; crochets on A3-A6, uniordinal, in transverse bands, about 11 per proleg, smaller in size laterally; on A10 equally uniordinal in single band (MacKay 1968, Delgado 2004). First three instars shiny yellow; fourth and fifth instars opaque yellow. Cephalic capsule brilliant reddish dark brown, smaller than prothorax, hypognathus. Body cylindrical, but A8 to A10 dorsally depressed. Head: With rounded superior (dorsal margin) from frontal aspect ( Fig. 8 View FIGURES 7 – 8 ); epicranial suture extending to less than one-half length of head; ecdysial suture and lateral adfrontal suture well defined, between them setae AF2 and pore AFa visible, between these sutures seta AF1 present on each side; F1 setae long, robust, pores (Fa), clearly visible; P2 short, robust; P1 very long, robust, inserted near level of adfrontal pores (AFa); A1and A2 similar in length, A3 very long; A1 near AF1 toward base of mandible, A2 below Pa, above A1; A3 located at sides of cephalic capsule, near L1, above stemmata; L1 long but never as long as A3 (located next to L1), robust; stemmatal seta 1 (S1) located between stemmata 2 and 4, short, robust; S2 more laterally located, next to stemmata 1; substemmatal setae 1(Ss1), long, robust, positioned ventrally, near base of mandible. Thorax and abdomen ( Fig. 5 View FIGURES 5 – 6 ): L group trisetose on T1; SV1 and SV3 inserted on side of prolegs on A3-A6; D1, D2, SD1, and L1 present on A7, latter two setae separated from each other in vertical line by oval spiracle; D1 and D2 dorsally on A8; large, oblique spiracles surrounded by L1, L2, L3, and SV1; D2 more dorsal and closer to segment A10 on A9, whereas D1 more lateral and closer to SD1, L1 and SV1 ventrally located; D2 positioned more apically, D1 more laterally, and L1 apically on A10 than on A9; V1 setae present.
Pupa ( Figs. 9, 10 View FIGURES 9 – 10 ): Typical of species in tribe Synanthedonini Niculescu and the genus Carmenta (see Mosher 1916, Eichlin & Passoa 1983); head with prominent tubercle; abdomen with segments movable, dorsally with two rows of spines on A3-A6 (spines of anterior row larger and more stout than those on posterior row), one row of spines on A7-A9 (except A7 with two rows on males only); cremaster absent, replaced by eight flattened, triangularly pointed spines, irregularly spaced in circular pattern.
Host plant. Chromolaena odorata (Asteraceae) .
Distribution. Found over a wide area from the northeastern Paria peninsula to the foothills of the Venezuelan Andes in the Trujillo region, Venezuela.
Holotype. Male, Venezuela, on Yaritagua side of Urachiche, before bridge over Urachiche River, 10°08.864'N, 69°00.283'W, 438 m (Wpt. VE 005), between Yaritagua and Urachiche, near timber factory, 10°04.924'N, 69°04.424'W, 416 m (Wpt. VE 006), 11 Nov 2005, L. Strathie, C. Zachariades, O. Delgado; AcCe 381, adult from field-collected larva, eclosion date: not recorded, died: 18 Jan 2006 ( MIZA).
Paratypes (9 males, 13 females): VENEZUELA: Yaracuy, via Yaritagua–Maracay, 10°04.799'N, 69° 04.607W, en Chromolaena odorata , 413 m, 9 Nov 2004 (1 female), emergió: 14-V-2005, Murió: 18-V- 2005, O. Delgado, L. Strathie, C. Zachariades, H. Diaz, PPRI-ARC-MIZA ( MIZA). Yaracuy, via Yaritagua– Maracay, 10°04.799'N, 69° 04.607W, en Chromolaena odorata , 413 m, 0 9 Nov 2004 (1 female), emergió: 17 May 2005, murió: 19 May 2005, O. Delgado, L. Strathie, C. Zachariades, H. Diaz, PPRI-ARC-MIZA; same locality, 9 Nov 2004 (1 female), emergió: 18 May 2005, murió: 23 May 2005 (no abdomen); same locality, 9 Nov 2004 (1 male), emergió: 23 May 2005, murió: 24 May 2005; same locality, 391 m, 10°04.472'N, 69° 05.712W, 9 Nov 2004 (1 female), emergió: 25 May 2005, murió: 28 May 2005. Yaracuy, via Barquisimento–Maracay, Hda. Sta. Lucia, 10°05.374'N, 69° 08.547W, 404 m, en Chromolaena odorata , 15 Dec 2004 (1 male), emergió: 29 May 2005, murió: 30 May 2005, O. Delgato, Q. Arias, PPRI-ARC-MIZA; same locality, 15 Dec 2004 (1 female), emergió: 30 May 2005, murió: 1 Apr 2005. Yaracuy, Yaritagua, via San Felipe, 10°04.799'N, 69° 04.607W, 413 m, 9 Nov 2005 (1 female), emergió: 19 Jan 2005, en Chromolaena odorata, O. Delgato, L. Strathie. On Yaritagua side of Urachiche (east-flowing), along freeway, before bridge over Urachiche River, 10°08.864'N, 69°00.283'W, 438 m (Wpt. VE 005), 11 Nov 2005 (1 male, 1 female), L. Strathie, C. Zachariades, O. Delgado; AcCe 381, Adult from field-collected larva, eclosion date: not recorded, died: 11 Jan 2006. Between Yaritagua and Urachiche just past Granja Elena farm & before garage, near timber factory, 10°04.924'N, 69°04.424'W, 416 m (Wpt. VE 006), 11 Nov 2005 (1 male), L. Strathie, C. Zachariades, O. Delgado; AcCe 381, adult from field-collected larva, eclosion date: not recorded, died: 13 Jan 2006. On Yaritagua side of Urachiche, before bridge over Urachiche river, 10°08.864'N, 69°00.283'W, 438 m (Wpt. VE 005), between Yaritagua and Urachiche, near timber factory, 10°04.924'N, 69°04.424'W, 416 m (Wpt. VE 006), 11 Nov 2005 (1 male), L. Strathie, C. Zachariades, O. Delgado; AcCe 381, adult from field-collected larva, eclosion date: 28 Dec 2005, died: 2 Jan 2006; same locality, 11 Nov 2005 (1 male, 1 female), eclosion date: not recorded, died: 11 Jan 06; same locality, 11 Nov 2005 (1 female), eclosion date: 12 Jan 2006, died: 13 Jan 2006; same locality, 11 Nov 2005 (1 male), eclosion date: 12 Jan 2006, died: 14 Jan 2006; same locality, 11 Nov 2005 (1 male, 1 female), eclosion date: 13 Jan 2006, died: 15 Jan 2006; same locality, 11 Nov 2005 (4 females), eclosion date: 14 Jan 2006, died: 17 Jan 2006. Paratypes deposited in SANC, CSCA, MIZA.
Etymology. The specific name, chromolaenae , is derived from the generic name of the host plant, Chromolaena odorata .
Biology. Under laboratory conditions, eggs of Carmenta chromolaenae are usually deposited singly or in pairs in the grooved upper surface of a petiole, near the junction of the main stem and terminal or axillary bud of C. odorata . Eggs frequently are deposited on both of oppositely positioned petioles, and generally at the upper few nodes. The highly mobile larva hatches from the ridged end of the egg positioned closest to the stem, tunnels into the shoot tip, boring down the stem a short distance before girdling it, which causes wilting and death of the shoot tip. The larva continues to bore down the stem in a narrow tunnel through the stem pith. Small holes are created by the larva at intervals along the stem, through which frass and stem pith extrude. After tunneling for a distance up to 30 cm down the stem and creating an emergence window by chewing the stem to leave only an epidermal layer, the larva pupates facing upwards within the stem. The emergence window is usually up to 6 cm higher up the stem than the end of the larval tunnel. The pupal case, assisted by the rows of spines on the abdominal segments, is typically partially extruded through the emergence window during emergence. Developmental duration from egg to adult is approximately two to three months during the warm, wet season, but individuals will diapause within stems during the dry season; consequently, the life cycle is more protracted (up to approximately six months).
Preliminary studies on the field host range of C. chromolaenae in Venezuela were conducted in 2005, examining seven Asteraceae species ( Aldama dentata La Llave & Lex , Bidens bipinnata L. Synedrella nodiflora (L.) Gaertn., Ageratum sp., Tridax procumbens L., Ve r b e s i n a sp. Viguiera sp.) growing in close proximity to Chromolaena plants, within areas where the moth was found to be abundant, and searching for damage caused by the moth. No damage was found on any of the other plant species examined (L. Strathie & C. Zachariades unpubl.). Host range testing on selected native and economically important species of Asteraceae is currently being conducted in quarantine in South Africa to further establish the host specificity status of C. chromolaenae to determine its safety for release as a biological control agent in South Africa.
Discussion. Larval damage caused by C. chromolaenae on C. odorata was first observed during field surveys in Venezuela in 1998 (Zachariades unpubl.). This damage was characterized by the presence of a small hole in the young stem below the axial pair of leaves, approximately 20 mm below which a girdle (due to larval tunneling just under the surface of the stem) was present, causing the shoot tip above to wilt and die ( Fig. 3 View FIGURES 1 – 4 ). Damage to the shoot-tip inflicted by C. chromolaenae resembles larval damage caused by the widespread shoot-tip mining fly Melanagromyza eupatoriella Spencer ( Diptera : Agromyzidae ) on C. odorata ; however, the fly causes a visible spiral mine down the shoot-tip, whereas the tunnel of C. chromolaenae is not externally visible. For oviposition, C. chromolaenae seems to favor large Chromolaena bushes in open, hot, exposed situations. Although C. chromolaenae was present at several sites in the northern regions (western, central and eastern) of Venezuela, it was locally abundant in only a few areas, particularly in the vicinity of Yaritagua, Yaracuy state (Zachariades & Strasthie unpub.).
A small number of C. chromolaenae larvae within stems were imported on several occasions from Venezuela into quarantine in South Africa, but a laboratory culture could not be established due to insufficient individuals collected, in combination with asynchronous eclosion of relatively short-lived adults that did not allow for mating opportunities. In late 2005 a large number (about 120) of moth larvae were collected in stems from areas near Yaritagua (Strathie unpubl.). Eclosed adults were exposed to a variety of conditions within the laboratory and glasshouse in attempts to induce mating and oviposition. This was successful in gauze cages of 0.5 x 0.5 x 1 m within the glasshouse, and subsequent generations were produced. This culture was supplemented by further collections of larvae from the Yaritagua area in late 2006.
This new species holds promise, both in terms of its biology and host range, as a potential biocontrol agent for C. odorata . In parts of South Africa and elsewhere, C. odorata has invaded regions that experience strongly seasonal wet and dry periods. Plants lose leaves and experience stem dieback or death during the dry seasons. This degenerated plant condition is not favorable for leaf feeders or agents originating from areas that do not experience distinct wet and dry periods. The northern parts of Venezuela, from where C. chromolaena is known, experience distinct dry periods similar to the climate in South Africa ( Robertson et al. 2008); so, the insect is able to tolerate these conditions, making it a suitable candidate for introduction into South Africa. The insect’s biology also enables it to diapause over the dry season, eclosing at the start of the following wet season.
Other Carmenta View in CoL species have been used successfully in weed biocontrol programs around the world, such as C. ithacae (Beut.) View in CoL on Parthenium hysterophorus View in CoL L. ( Asteraceae View in CoL ) ( Withers et al. 2000). A root-boring species from Argentina and Chile, C. haematica (Ureta) View in CoL , has been recommended for the control of snakeweeds, Gutierrezia View in CoL spp. ( Asteraceae View in CoL ) in the U.S.A. ( DeLoach 1980). Another stem-boring species, C. mimosa Eichlin & Passoa (1983) View in CoL , was released in Australia in 1989 ( Steinbauer 1998) to help control the invasive plant, Mimosa pigra View in CoL L. ( Fabaceae View in CoL ). In addition, an African vine borer, Melittia oedipus Oberthür View in CoL , has been evaluated for its potential to control ivy gourd ( Coccinia grandis View in CoL (L.) Voigt; Cucurbitaceae View in CoL ), a weed pest in Hawaii ( Eichlin 1995).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Carmenta chromolaenae Eichlin
| Eichlin, Thomas D., Delgado, Oona S., Strathie, Lorraine W., Zachariades, Costas & Clavijo, Jose 2009 |
C. mimosa
| Eichlin & Passoa 1983 |