Laonice irinae Sikorski, Radashevsky & Nygren, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4996.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:9FF4827B-A424-4D02-A58D-2CB37E1FAB5A |
|
persistent identifier |
https://treatment.plazi.org/id/0EC029F6-7EAA-4FC5-B1A2-643D4D405DC1 |
|
taxon LSID |
lsid:zoobank.org:act:0EC029F6-7EAA-4FC5-B1A2-643D4D405DC1 |
|
treatment provided by |
Plazi |
|
scientific name |
Laonice irinae Sikorski, Radashevsky & Nygren |
| status |
sp. nov. |
Laonice irinae Sikorski, Radashevsky & Nygren View in CoL n. sp.
http://zoobank.org:act: 0EC029F6-7EAA-4FC5-B1A2-643D4D405DC1
( Figs 5B View FIGURE 5 , 6 View FIGURE 6 , 7 View FIGURE 7 , 8 View FIGURE 8 , 9 View FIGURE 9 , 15A View FIGURE 15 , 18B View FIGURE 18 , 19A View FIGURE 19 , Table 3)
Laonice sarsi Söderström, 1920 (Part.) View in CoL : 223–225, figs 129, 130.
Laonice cirrata: Ditlevsen 1929: 29 View in CoL . Kirkegaard 1969: 76, fig. 40. Not M. Sars 1851.
Laonice bahusiensis: Sikorski 2003 (Part.) View in CoL : 320–325, figs 3A–I, 4A–B, 5A–B, 6F. Not Söderström 1920.
Type locality. NORWAY, Norwegian Sea , Mikkelsøya, st. C2-1, 68.6559°N, 14.7293°E, 38 m. GoogleMaps
Type material. ZMBN 135386 View Materials (holotype), 116570, 135363–135385, 135387–135394, 135398, 136063– 136067 (69 paratypes) ; MIMB 39043–39046 View Materials (42 paratypes) ; NHMD 108539 , 108540 , 108546 (4 paratypes) ; UUZM 52581 View Materials , 52582 View Materials (2 paratypes) .
Adult morphology. Up to 45 mm long, 1.5 mm wide for 110 chaetigers; smallest examined complete individual 10 mm long, 0.3 mm wide for 60 chaetigers. Holotype 39 mm long, 1 mm wide for 102 chaetigers. Pigmentation absent on body and palps.
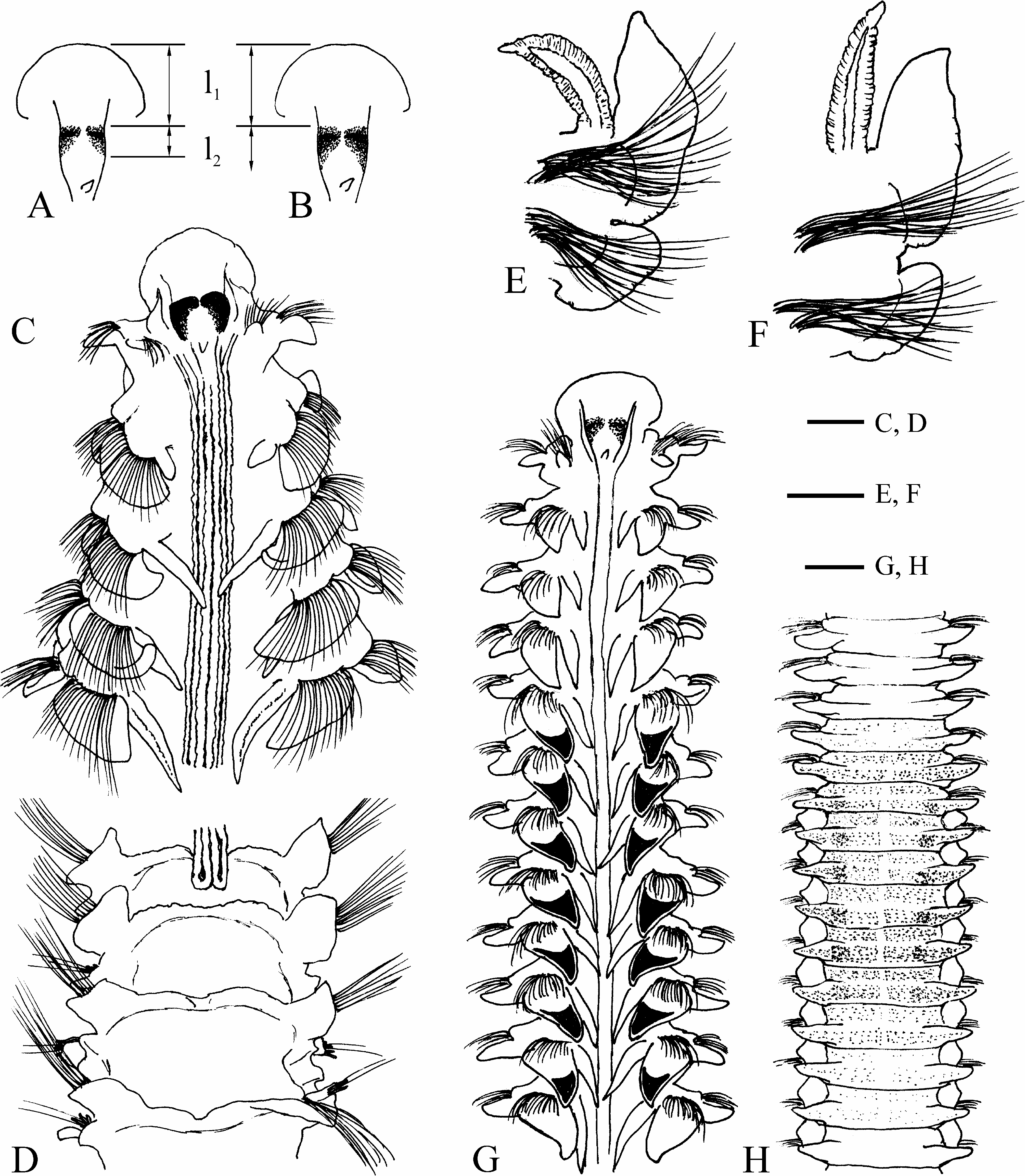
Prostomium approximately triangular, anteriorly wide, usually broadly rounded, occasionally truncate to slightly concave, fused with fronto-lateral margin of peristomium ( Figs 6A, G View FIGURE 6 , 7A View FIGURE 7 ), posteriorly extending over 36 chaetigers (to end of chaetiger 32 in holotype) as a low narrow caruncle, shorter in small individuals ( Fig. 8A View FIGURE 8 ). Nuchal organs U-shaped ciliary bands on sides of caruncle ( Figs 6A View FIGURE 6 , 7A View FIGURE 7 ). Length of nuchal organs was strongly correlated with individual number of branchiate chaetigers ( Fig. 8C, r View FIGURE 8 2 View FIGURE 2 = 0.8927, n = 57). Occipital antenna usually well developed ( Figs 6A View FIGURE 6 , 7A View FIGURE 7 ). Two pairs of red eyes (appearing almost black in formalin-fixed specimens) arranged trapezoidally, comprising one pair of large median eyes and one pair of small lateral eyes situated slightly anteriorly and set wider apart (often close to boundary between prostomium and peristomium and therefore not visible in dorsal view) ( Fig. 6A View FIGURE 6 , 15A View FIGURE 15 ). Palps as long as 5–12 chaetigers, with deep frontal longitudinal groove lined with cilia.
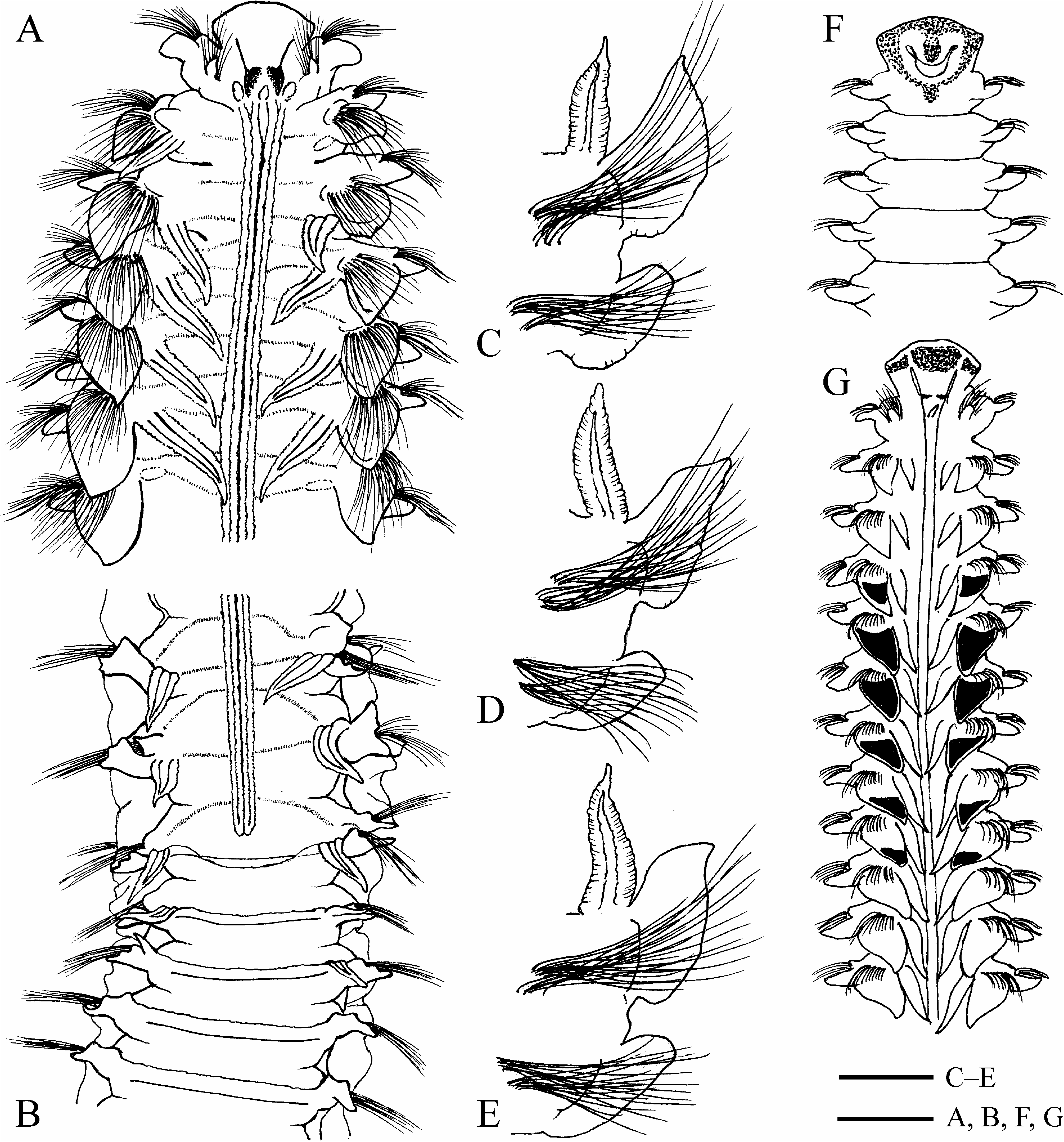
Chaetiger 1 with well-developed capillary chaetae and small postchaetal lamellae in both rami; notopodial lamellae triangular; neuropodial lamellae rounded.All notopodia with capillary chaetae only. Low prechaetal lamellae present in noto- and neuropodia on anterior chaetigers after chaetiger 1. Notopodial postchaetal lamellae large, leaf-like on branchiate chaetigers, usually largest on chaetiger 4, not overlapping middorsally, greatly diminishing in size on posterior abranchiate chaetigers; lamellae on at least ten anterior chaetigers with terminal acute peaks on upper tips ( Fig. 6A, C–E, G View FIGURE 6 ). Neuropodial postchaetal lamellae ear-like on branchiate chaetigers, greatly diminishing in size on posterior abranchiate chaetigers.
Branchiae from chaetiger 2, up to 32 pairs (on chaetigers 2–30 in holotype); first pair shorter or similar in length to notopodial postchaetal lamellae of chaetiger 2; from chaetigers 5–6 branchiae longer (up to twice as long) than notopodial postchaetal lamellae ( Fig. 6E, G View FIGURE 6 ), gradually diminishing in size on succeeding chaetigers, posteriorly disappearing 0–6 (usually 1–3) chaetigers before end of caruncle and nuchal organs, or one chaetiger after end of nuchal organs ( Figs 6B View FIGURE 6 , 7B View FIGURE 7 , 8A, C View FIGURE 8 , 19A View FIGURE 19 ). Individual number of branchiae was strongly correlated with length of nuchal organs ( Fig. 8C View FIGURE 8 ).
Dorsal transverse crests one per chaetiger, beginning 1–4 chaetigers before, and arranged on up to 15 chaetigers after the posterior end of nuchal organs ( Figs 6B View FIGURE 6 , 7B View FIGURE 7 ). Several anterior dorsal crests interrupted by nuchal organs (nuchal organs sometimes are interrupted by the dorsal crests on one or two most posterior chaetigers); on succeeding chaetigers crests continuous, interconnecting notopodial postchaetal lamellae ( Fig. 6B View FIGURE 6 ).
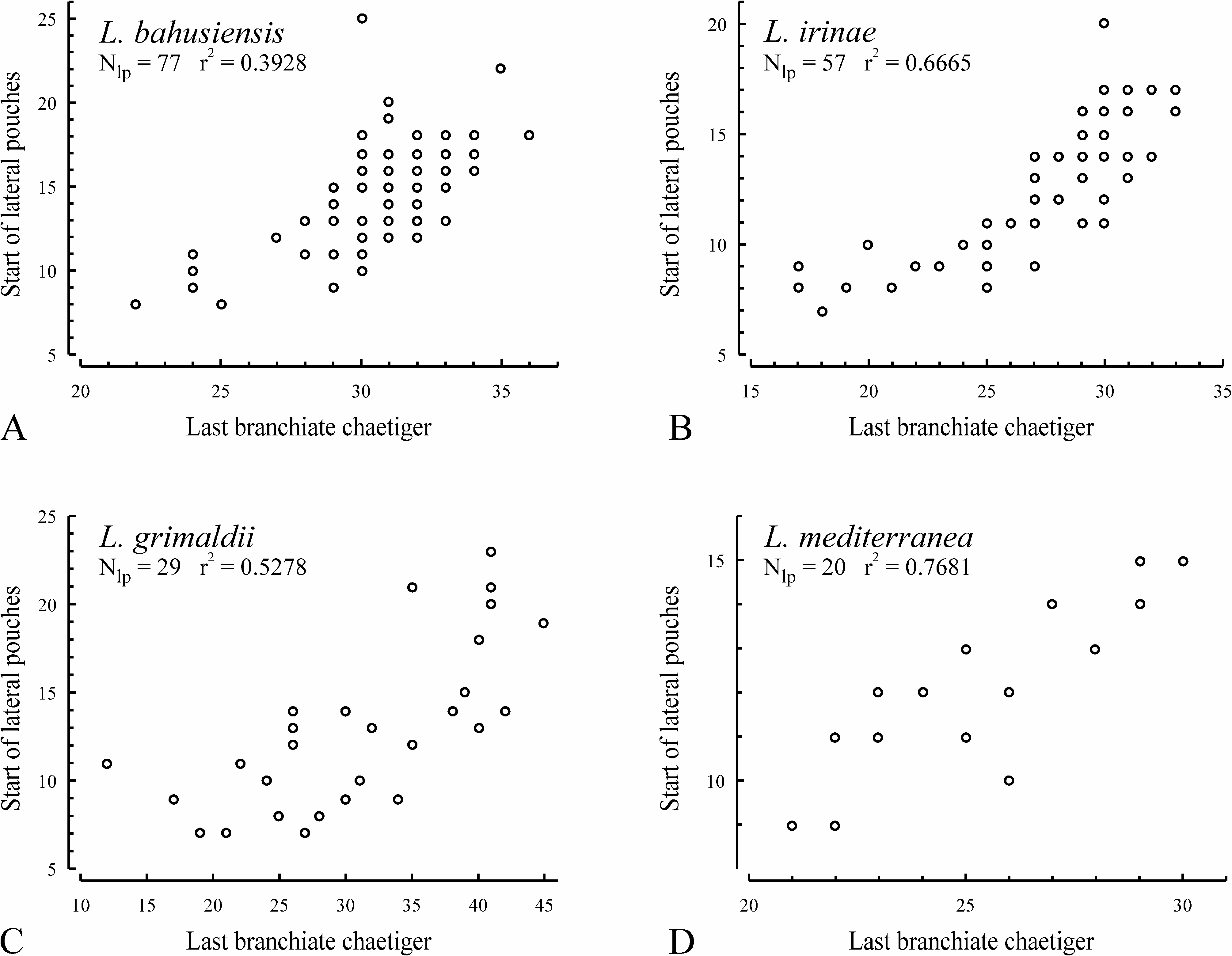
Lateral interneuropodial pouches from chaetigers 6–20 (from chaetiger 13 in holotype) to end of body. Anterior start of pouches was moderately correlated with individual number of branchiate chaetigers ( Fig. 18B, r View FIGURE 18 2 View FIGURE 2 = 0.6665, n = 57).
Sabre chaetae in neuropodia from chaetigers 9–21 (from chaetiger 17 in holotype), from more anterior chaetigers in small individuals ( Fig. 8B View FIGURE 8 ), 1–2 in a tuft; chaetae with weak granulation on shaft, up to twice as long as hooks. Anterior start of sabre chaetae was moderately correlated with individual number of branchiate chaetigers ( Fig. 8D, r View FIGURE 8 2 View FIGURE 2 = 0.5057, n = 58).
Hooded hooks in neuropodia from chaetigers 14–35 (from chaetiger 29 in holotype), from more anterior chaetigers in small individuals ( Fig. 8B View FIGURE 8 ), up to 10 in a series, accompanied by inferior sabre chaetae and alternating capillaries throughout body. Alternating capillaries with very narrow limbation, up to 1.5 times as long as hooks, up to 10 in a series, in middle and posterior chaetigers situated in upper part of hook row. Hooks quinquedentate, with two pairs of upper teeth arranged in two vertical rows above main fang ( Fig. 7C View FIGURE 7 ), or quadridentate, with one pair of upper teeth and a single superior median tooth above main fang; shaft slightly curved, slightly narrowed at level of body cuticle ( Fig. 9F View FIGURE 9 ). Anterior start of hooks was strongly correlated with individual number of branchiate chaetigers ( Fig. 8D, r View FIGURE 8 2 View FIGURE 2 = 0.8138, n = 58).
Pygidium with up to eleven pairs of cirri (seven pairs in holotype) arranged around terminal anus, comprising one pair of ventral cirri and up to ten pairs of thinner and longer dorsal cirri; fewer cirri in small individuals.
Digestive tract without gizzard-like structure.
Nephridia from chaetiger 4 to chaetigers 21–24, present in all anterior sterile chaetigers except chaetigers 1–3, fewer in small individuals.
Reproduction. Laonice irinae n. sp. is dioecious. Gametes develop in both females and males from chaetigers 23–25 through most of the body. Oogenesis is entirely intraovarian; vitellogenesis occurs when oocytes grow while attached to segmental blood vessels ( Fig. 9B View FIGURE 9 ). Developed oocytes are released into the coelomic cavity and accumulate in the coelom until spawning. The largest intraovarian oocytes were oval, up to 205x 275 µm in diameter, with the germinal vesicle about 80 µm and a single nucleolus 25 µm in diameter. The oocyte envelope was about 15 µm thick, with a honey-combed sculptured external surface, and pear-shaped cortical alveoli arranged in two parallel complete circles and one oblique incomplete row situated between the circles ( Fig. 9A–D View FIGURE 9 ). The large circle comprised 14–17 alveoli situated close to the attachment place of the oocyte to the blood vessel ( Fig. 9B, C View FIGURE 9 ). The small circle comprised 7–9 alveoli ( Fig. 9A, B View FIGURE 9 ), while the intermediate row comprised about 10 alveoli ( Fig. 9A–C View FIGURE 9 ). Each alveolus was up to 20 µm deep, with an external opening about 10 µm in diameter, and an inner widest part up to 14 µm in diameter. When artificially released into sea water, the oocytes became spherical ( Fig. 9D View FIGURE 9 ).
Spermatogonia proliferate in the testes; spermatogenesis occurs in the coelomic cavity. Spermatids were joined in tetrads. Spermatozoa were ect-aquasperm with a small rounded acrosome, a spherical nucleus about 3 μm in di- ameter, spherical mitochondria, probably four in number and each one less than 1 µm in diameter, and a flagellum about 87 µm long ( Fig. 9E View FIGURE 9 ).
Methylene green staining. Intensely stained upper parts of frontal surfaces of notopodial postchaetal lamellae from chaetiger 2 to approximately chaetiger 9 ( Fig. 6G View FIGURE 6 ). Usually no staining on prostomium, peristomium and ventral side of chaetigers; rarely, intensely stained mid-longitudinal line in anterior half of prostomium and a spot posterior to occipital antenna, and diffusely stained dorsal and ventral sides of anterior parts of prostomium and peristomium (except the most frontal edge) ( Fig. 6F, G View FIGURE 6 demonstrates a rare case of intense staining).
Remarks. The specimens herein referred to L. irinae n. sp. had earlier been misidentified as L. bahusiensis ( Sikorski 2002, 2003). However, in L. bahusiensis the nuchal organs terminate 0–10 chaetigers before the last branchiate chaetiger ( Fig. 19A View FIGURE 19 ), and the dorsal transverse crests first appear on a chaetiger after the end of the nuchal organs, whereas in L. irinae n. sp. the nuchal organs usually extend beyond the last branchiate chaetiger ( Fig. 19A View FIGURE 19 ), and the dorsal transverse crests first appear 1–5 chaetigers before the end of the nuchal organs ( Fig. 6B View FIGURE 6 ).
Some individuals morphologically similar to L. irinae n. sp. were collected on the shelf of Portugal (material deposited at the Biological department of the University of Aveiro, Portugal). Considering the long distance from the southern distributional limits of L. irinae n. sp. reported here, and lacking molecular support, we decided to postpone their final identification.
Etymology. The species is named in honour of Irina Sikorskaya , wife of the first author.
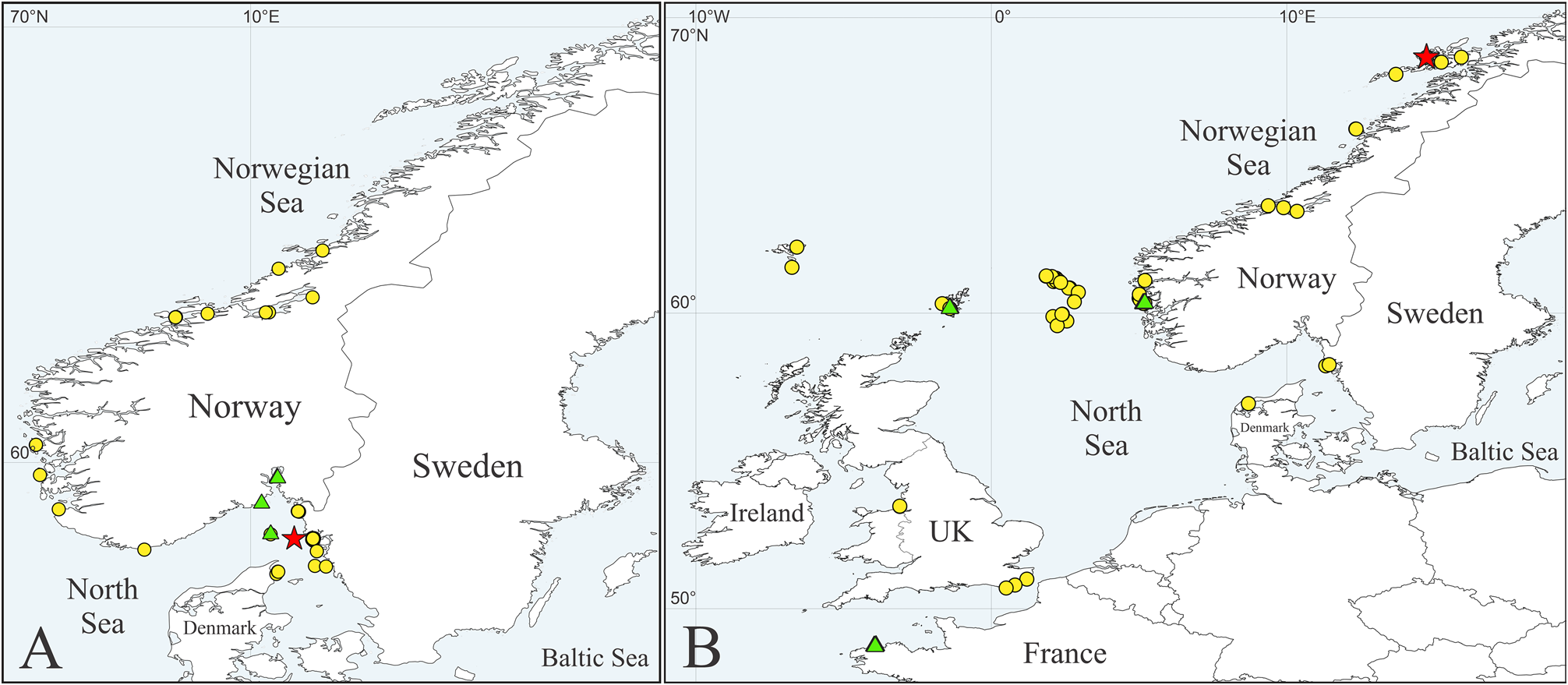
Distribution. Faroe Islands; Shetland Islands; Norwegian Sea, from Bogelva (68.7°N) south to the North Sea, Gullmarfjord, Sweden, and Limfjord, Denmark; English Channel; Irish Sea, Liverpool Bay; Ireland, Deenish Island ( Fig. 5B View FIGURE 5 ). At 4–156 m depth.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Laonice irinae Sikorski, Radashevsky & Nygren
| Sikorski, Andrey V., Radashevsky, Vasily I., Castelli, Alberto, Pavlova, Lyudmila V., Nygren, Arne, Malyar, Vasily V., Borisova, Polina B., Mikac, Barbara, Rousou, Maria, Martin, Daniel, Gil, João, Pacciardi, Lorenzo & Langeneck, Joachim 2021 |
Laonice cirrata:
| Kirkegaard, J. B. 1969: 76 |
| Ditlevsen, H. 1929: 29 |