Macrothrix hirsuticornis Norman and Brady, 1867
|
publication ID |
https://doi.org/ 10.1080/00222930701689937 |
|
persistent identifier |
https://treatment.plazi.org/id/03E32C46-B162-FFBF-FEE0-FC41D9A610BB |
|
treatment provided by |
Felipe |
|
scientific name |
Macrothrix hirsuticornis Norman and Brady, 1867 |
| status |
s. str. |
Macrothrix hirsuticornis Norman and Brady, 1867 View in CoL s. str.
( Figures 21 View Figure 21 , 22 View Figure 22 )
Macrothrix hirsuticornis Norman and Brady 1867, p 10 View in CoL –11, Plate 23, Figures 6 View Figure 6 , 7 View Figure 7 .
See references to many other descriptions from the Palaeactic in Smirnov (1976, 1992) and Flössner (1972, 2000).
Type locality
‘‘A slowly-running stream at Ashburn, Sunderland’’, England, UK.
Type material
Lectotype: a female in bad condition from ‘‘Ashborne, Sunderland’’, Norman’s Collection, NHM 1911.11.8.M.4038.
Material examined
Germany: a temporary water pool, Juist Island, East Frisian Islands , Lower Saxony, coll. 26 June 1987 by W. Hollwedel, AAK 1999-011 . Iceland: locality # 27, coll. 16 July 1996 by A. Thiery, NMK 1873 View Materials ; locality # 7, 30 km from Stadur, coll. 28 June 1996 by A. Thiery, NMK 1875 View Materials . Italy: Lake Campo Felice , Abruzzi, coll. 1982 by F. Margaritora, AAK 1999-084 . Israel: a pool in Dor, coll. 23 January 1967 by N. N. Smirnov, NNS 1997-045 . Iraq: a channel, Euphrates at Chybayish, coll. 18 November 1974 by N. N. Smirnov, NNS 1998-196 . Russia: Farm Urozhayniy, near Lake Svetloe , Altai Territory, coll. 18 August 1967, NNS 1997-246 ; Teletskoe Lake , Altai Territory, coll. 12 August 2002 by O. S. Burmistrova, NMK 2514 View Materials ; Irkutsk water Reservoir , Irkutsk Area, coll. 24 June 1968, NNS
1997-178, NNS MGU 1949, and NNS MGU 2188. Tajikistan: Lake Solongul-Kul , Central Pamirs, coll. 9 August 1948, NNS 1997-036 .
Short diagnosis
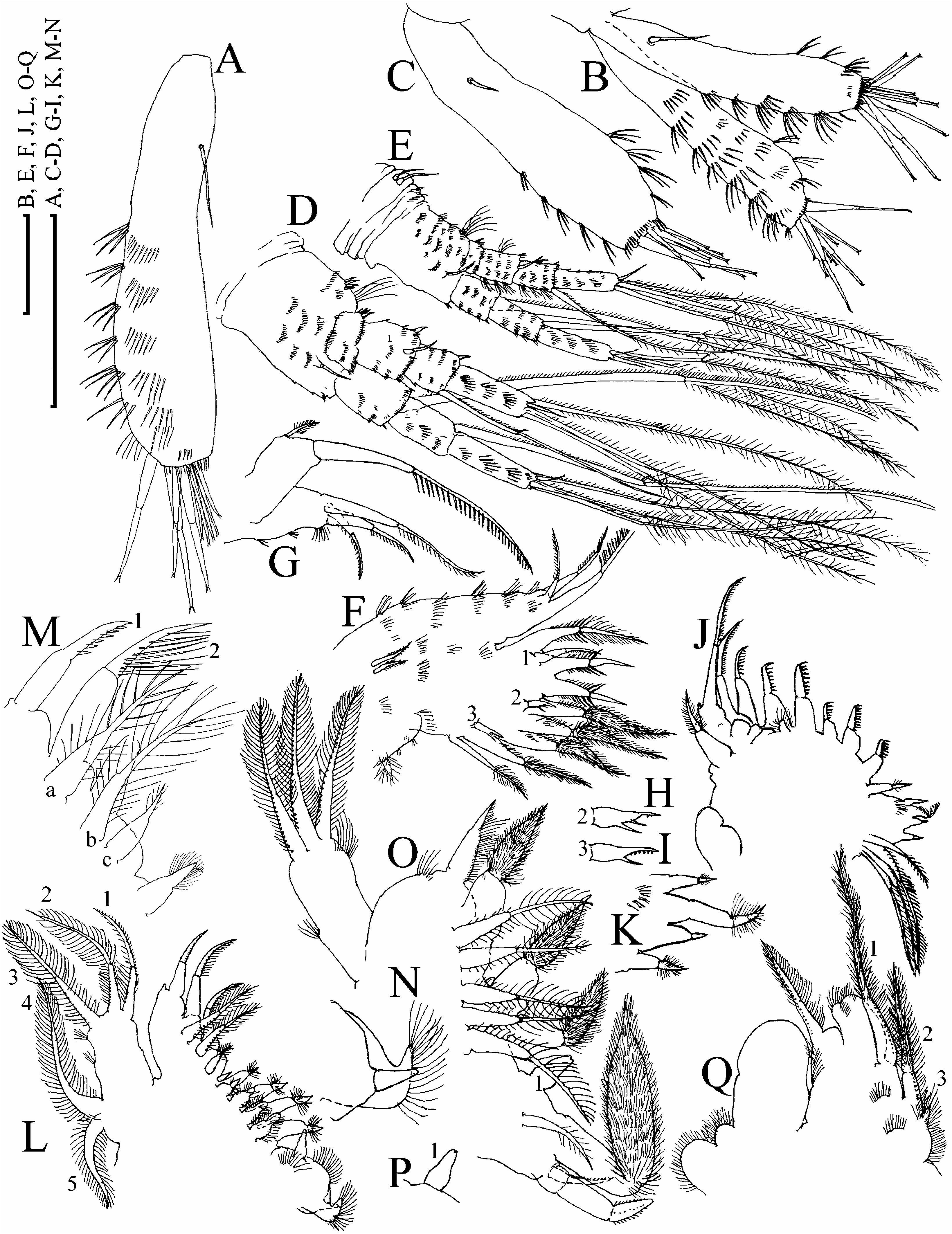
Parthenogenetic female. In lateral view body subovoid, cervical depression absent, dorsal margin breached by a ‘‘step’’ in posterior boundary of head, or not breached; posterodorsal angle as rounded triangle, lies in level of middle of body height or ventrally to it ( Figure 21A, B View Figure 21 ). No dome above eye. Ocellus small. Dorsal organ ovoid, small. Labrum with a moderately projected apex, with or without ill-defined tubercles ( Figure 21C View Figure 21 ). Armature of valve margin as in previously described species ( Figure 21D–H View Figure 21 ).
Postabdomen subovoid, with rounded distal extremity, without ‘‘heel’’ basally, and without a reticulation on sides or with poorly observable reticulation ( Figure 21I View Figure 21 ). Ventral margin straight, with series of fine setules ( Figure 21J View Figure 21 ). Dorsal margin distinctly bilobed; preanal margin with transversal series of minute setules, anal margin with groups of thicker setules. Postabdominal seta with distal segment densely armed with relatively long setules; basal segment with numerous, shorter setules ( Figure 21I View Figure 21 ). On external side of postabdominal claw, a series of 8–12 thin spinules ( Figure 21K View Figure 21 ); medial row of about seven to ten denticles; inner row with numerous denticles, organized in two successive series, subdivided by a larger denticle ( Figure 21L View Figure 21 ).
Antenna I widened distally, straight or slightly curved, without a subapical external angulation; sensory seta at distance of about two antennular diameters (at base) from antenna I joint; on anterior face about six to eight transverse rows of spinules, but no reticulation ( Figure 22A, B View Figure 22 ). Nine short aesthetascs, three of them significantly larger than the rest. Antenna II with distal spine on basal segment longer than proximal segment of exopod ( Figure 22D View Figure 22 ). Lateral seta on proximal endopod segment larger than other setae, lacking robust denticles in middle ( Figure 21O View Figure 21 ). A spine on second segment of exopod longer than half next segment. On posterior side of segments 1–3 of exopod there are series of small to large additional denticles.
Limb I with longest apical seta of outer distal lobe having distal segment unilaterally armed with robust setules, inner-distal lobe with three bisegmented setae of different size, unilaterally setulated in distal part, smallest one with whole distal segment setulated; two ejector hooks of similar size, a vestige of gnathobase I with a single fully setulated seta ( Figure 22F View Figure 22 ), anterior setae as represented in Figure 22H, I View Figure 22 . On limb II, scrapers 1–2 with delicate feathering, scrapers 3–7 with robust denticles of size characteristic for the genus; a solitary posterior seta near gnathobase; filter plate II with four setae, without a small hillock, a rudiment of fifth seta ( Figure 22J, K View Figure 22 ). On limb III epipodite with a distal group of three long setae, seta 1 shortest, distally armed with relatively fine setules ( Figure 22L View Figure 22 ); setulated projection proximally to seta 3; on inner-distal limb portion, seta 1 distally with short and robust denticles; seta a with fine setules basally and robust spinules distally, seta b long, seta c short ( Figure 22M View Figure 22 ); basal endite posteriorly with four soft posterior setae, gnathobase as in other species ( Figure 22N View Figure 22 ). Limb IV with exopodite small, bearing only a distal group of three bilaterally feathered setae of subequal size; on inner-distal portion of this limb, seta 1 without strong denticles distally; posteriorly row of five long setae ( Figure 22O View Figure 22 ), seta 1 on gnathobase as a bottle-shaped sensillum ( Figure 22P View Figure 22 ). On limb V there are three setae at inner margin ( Figure 22Q View Figure 22 ).
Juvenile female. Subquadrangular in shape, with relatively longer antenna I and II ( Figures 21M, N View Figure 21 , 22C, E View Figure 22 ).
Ephippial female. Similar to parthenogenetic female, with slightly pigmented ephippium bearing a slight additional sculpture of fine polygonal reticulation, no clear border between ephippium and rest of valve, dorsal wall of carapace forms a special dark, chitinized plate; normally two eggs, sometimes three in ephippium ( Berg 1933; Hudec 1983).
Male. Previous descriptions ( Smirnov 1976; Hudec 1983; Alonso 1996; Silva- Briano 1998) insufficiently accurate in fine details to discuss differences from congeners.
Size. Parthenogenetic females 0.5–2 mm, ephippial females 0.5–0.65 mm, males 0.4– 0.65 mm ( Flössner 2000).
Taxonomic notes. Initial examination of a relatively limited set of samples with Macrothrix from the Palaearctic (A. A. Kotov, unpublished) revealed three hirsuticornis -like taxa: M. hirsuticornis Norman and Brady, 1867 , M. tripectinata Weisig, 1934 , and M. dadayi Behning, 1941 . Silva-Briano (1998) redescribed M. hirsuticornis s. str. in his PhD thesis, but these results are not published. It is necessary to note that although Silva-Briano (1998) concluded that M. cornuta is a ‘‘horned’’ morphotype of M. hirsuticornis , the female of the former species from Turkey (whose identity with Daday’s (1903) species from Mongolia must be checked!) has strong spines on the seta on the proximal segment of the endopod of antenna II. Most probably the M. cornuta of Silva-Briano (1998) is another Palaearctic hirsuticornis -like species, but it is clear that the revision of M. hirsuticornis in the Palaearctic still has some way to go and must be done using better material.
Previous efforts by taxonomists examining the M. hirsuticornis group have predominantly concentrated on the variability of characters regarded as helpful for systematics by cladocerologists of the 19th century ( Sars 1890; Lilljeborg 1901). These include the expression of a tooth or hood on posterior border of head, or additional spines on antennal branches ( Fox 1962; Flössner 1967, 1972; Hudec 1983; Margaritora and Usai 1983; Silva- Briano 1998; Flössner 2000). All of these publications, dealing with external shape of body, lacked a detailed analysis of other, ‘‘finer’’, traits of different populations. Only after such accurate examination of many populations may a conclusion be made on the number of hirsuticornis -like species in the Palaearctic.
In any case, all southern hemisphere species described above differ from Palaearctic M. hirsuticornis s. str. ( Table I).
Distribution. Widely distributed in the Palaearctic. Status of Nearctic and all southern hemisphere populations must be checked.
Phylogeny
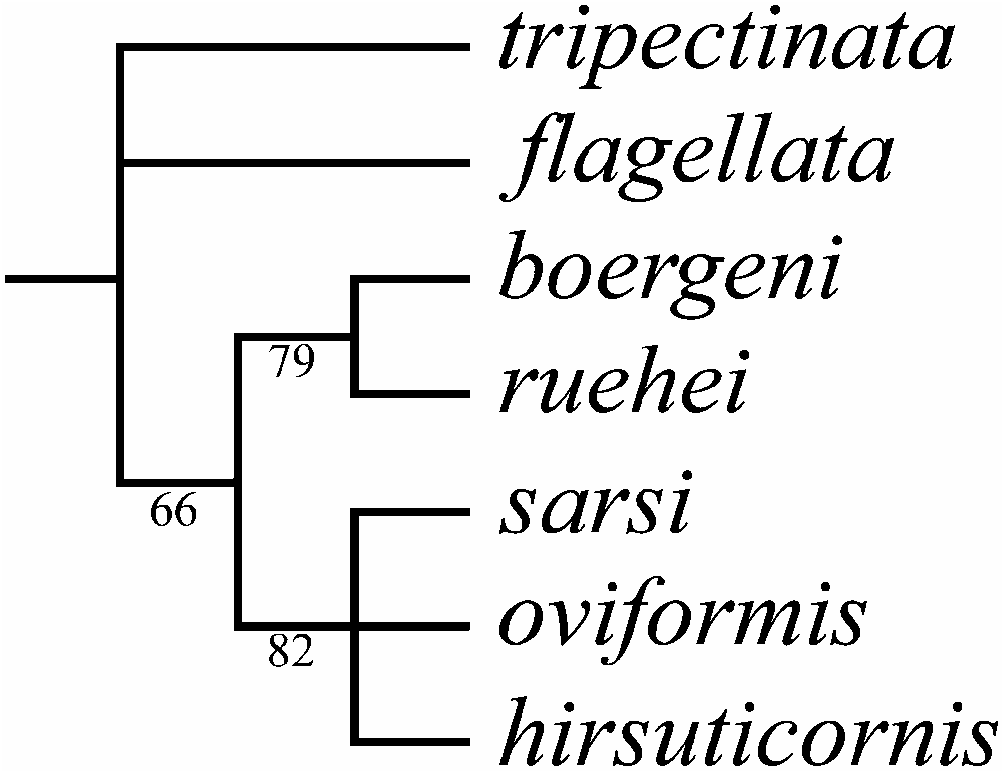
A list of characters analysed is given in Table I. Autapomorphies, marked by bold type, were excluded from the analysis; each variable character is marked as data missing. A cladistic search using 12 characters reveals three equally short trees (TL516; CI50.75; RI50.71); a strict consensus tree is represented in Figure 23 View Figure 23 . The 50% majority rule bootstrap simulation led to a tree of similar topology with the consensus tree. Due to this fact, branch probabilities were assigned to the aforementioned consensus tree.
Two well-supported clades are found: boergeni – ruehei and sarsi – oviformis – hirsuticornis ; these are grouped together, but this grouping has a moderate support (66%). Macrothrix flagellata is not grouped with any other taxa; this species as well as M. tripectinata (the outgroup) are basal members of the studied group.
| V |
Royal British Columbia Museum - Herbarium |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Macrothrix hirsuticornis Norman and Brady, 1867
| Kotov, Alexey A. 2007 |
Macrothrix hirsuticornis
| Norman AM & Brady GS 1867: 10 |