Neoseiulus barkeri Hughes, 1948
|
publication ID |
https://doi.org/10.11646/zootaxa.4500.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:16A34E21-D55D-40E9-BF2D-43D3BD8A6AF2 |
|
persistent identifier |
https://treatment.plazi.org/id/03E987BF-FFDF-FFEC-FF44-FA81FC1CE32C |
|
treatment provided by |
Felipe |
|
scientific name |
Neoseiulus barkeri Hughes, 1948 |
| status |
|
Neoseiulus barkeri Hughes, 1948 View in CoL
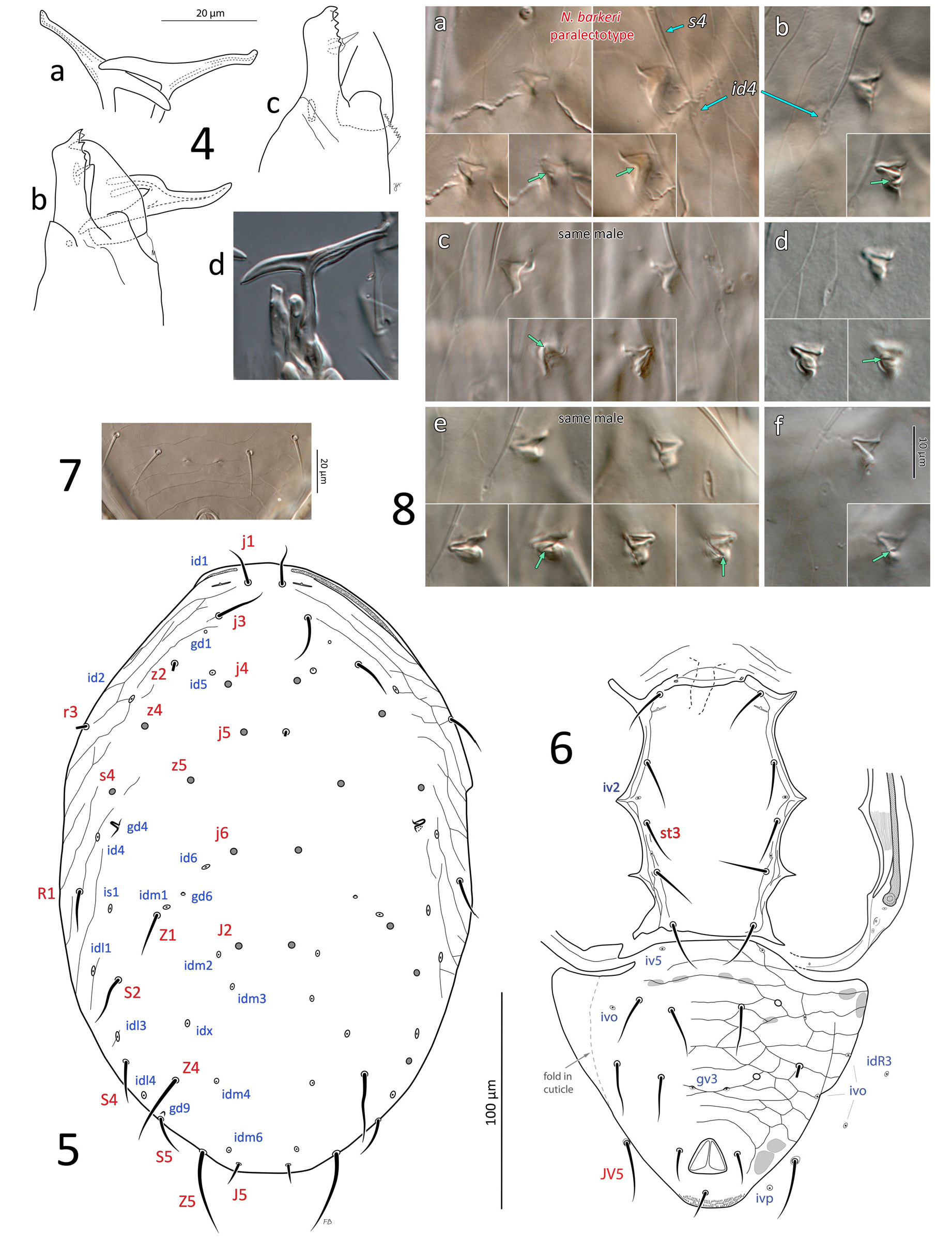
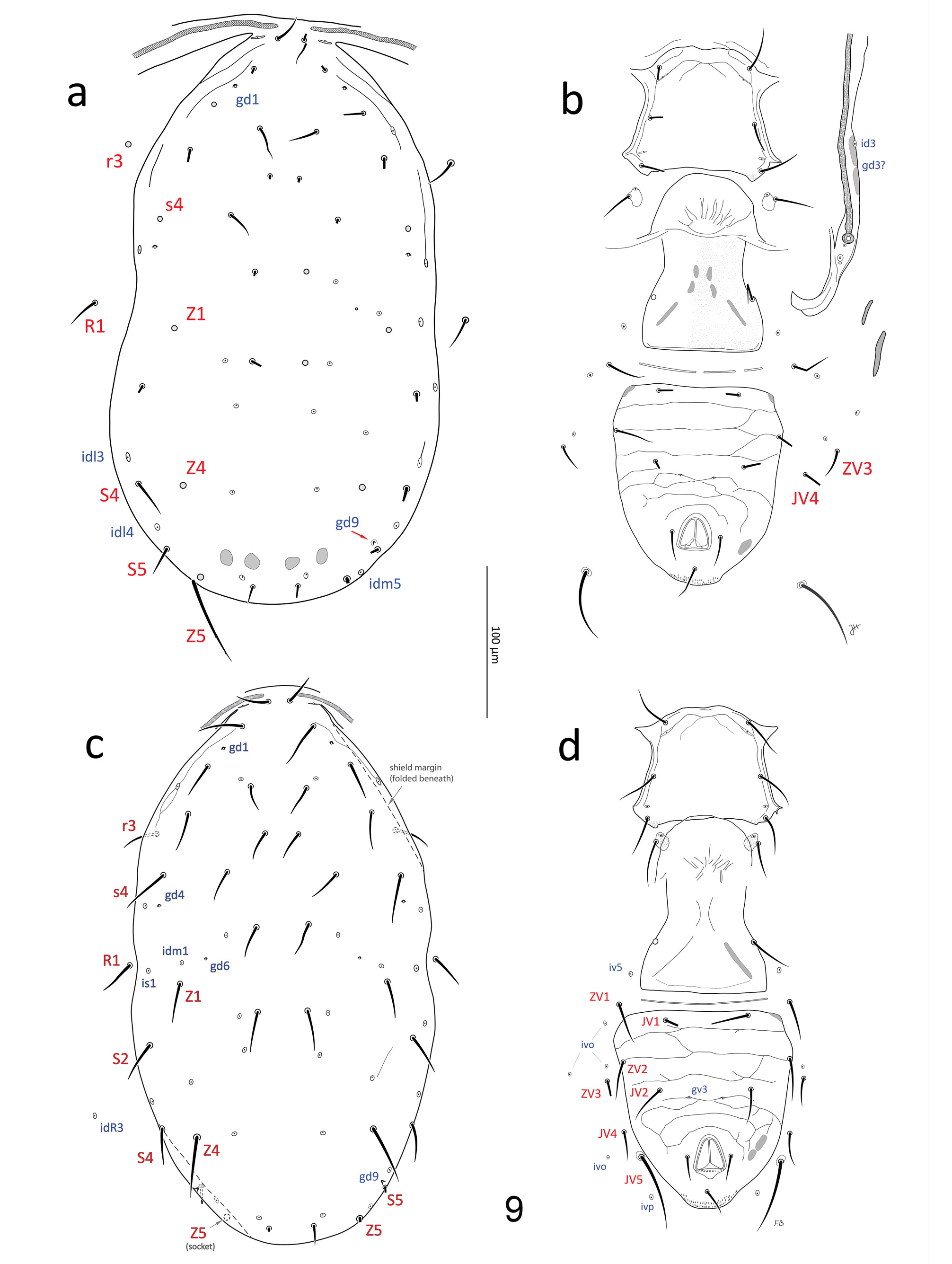
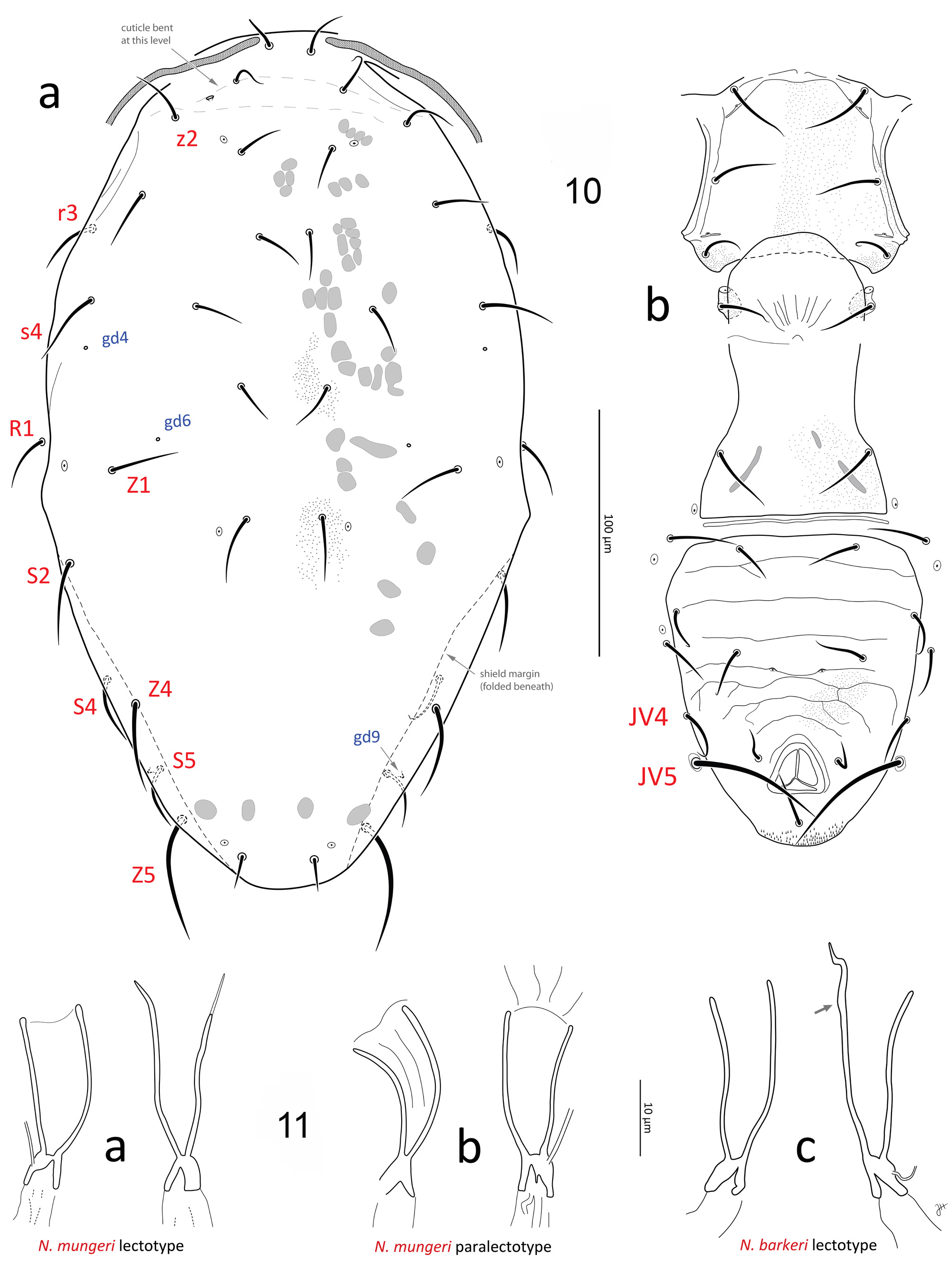
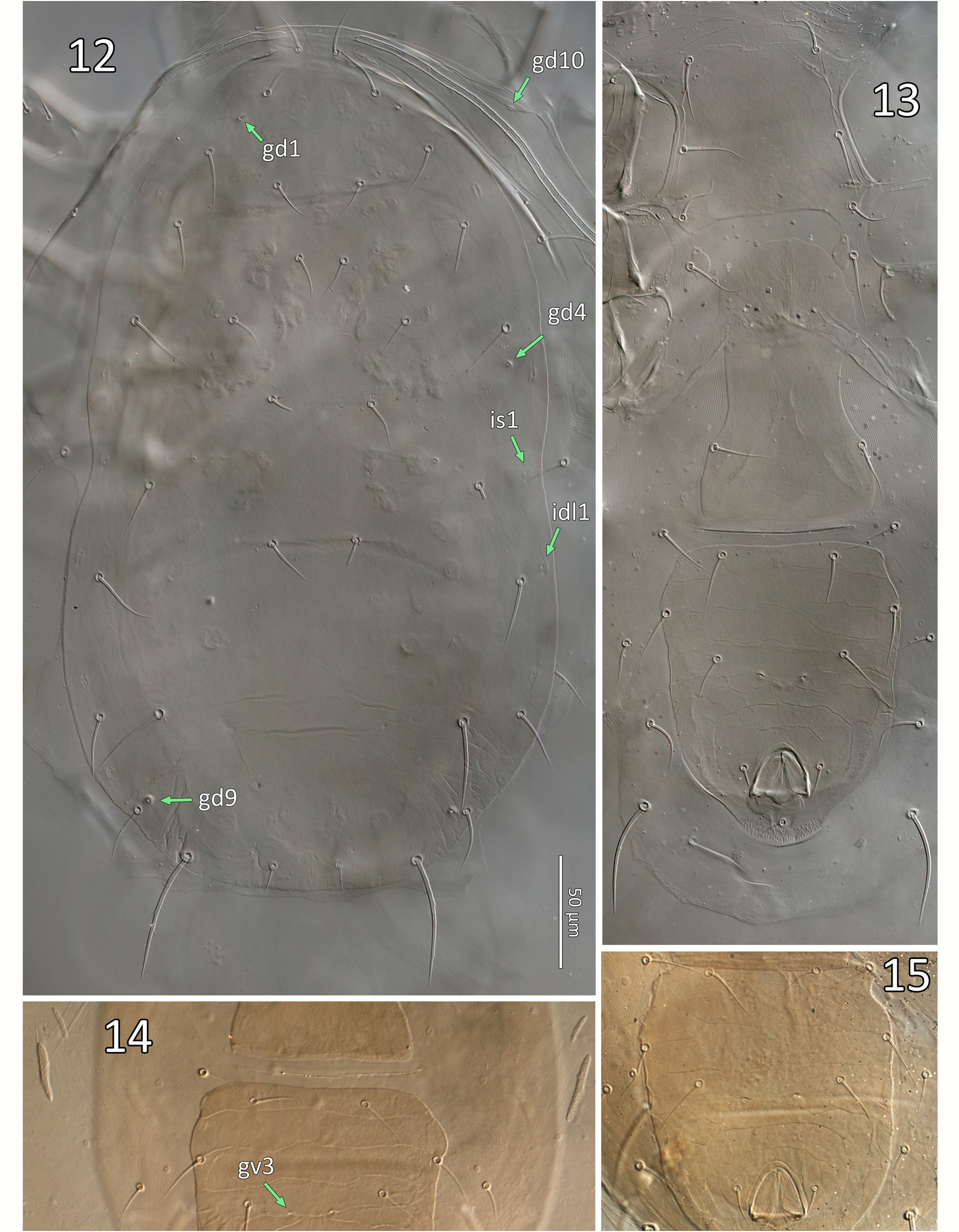
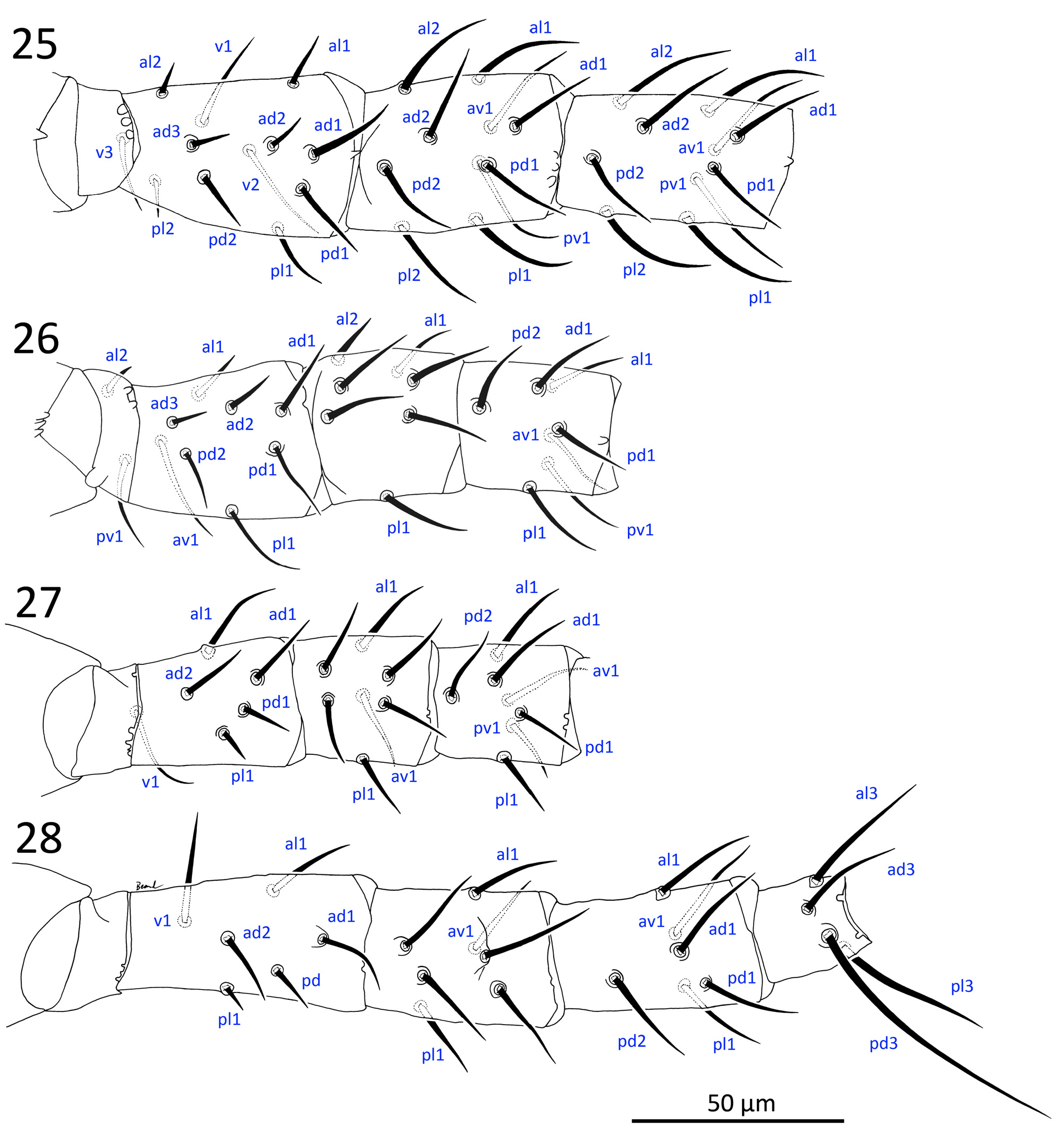
( Figures 2a–e View FIGURE 2 , 3–17 View FIGURE 3 View FIGURES 4–8 View FIGURE 9 View FIGURES 10–11 View FIGURES 12–15 View FIGURE 16 View FIGURE 17 , 19, 21, 23 View FIGURES 19–24 , 25–28 View FIGURES 25–28 ; Table 2)
Neoseiulus barkeri Hughes, 1948: 142 View in CoL .
Typhlodromus californicus McGregor, 1954: 89 . ( holotype examined). New synonymy, but usage of this name should be maintained.
Typhlodromus mungeri McGregor, 1954: 92 . ( syntypes examined). New synonymy.
Amblyseius mckenziei Schuster & Pritchard, 1963: 268 . ( holotype examined).
Amblyseius oahuensis Prasad, 1968: 1518 . ( paratypes examined).
Amblyseius picketti Specht, 1968: 681 . ( holotype examined).
Amblyseius cydnodactylon Shehata & Zaher, 1969: 177 . (based on literature alone).
Amblyseius mycophilus Karg, 1970: 290 . (based on literature alone).
Neoseiulus kermanicus Daneshvar, 1987: 14 View in CoL . (based on literature alone).
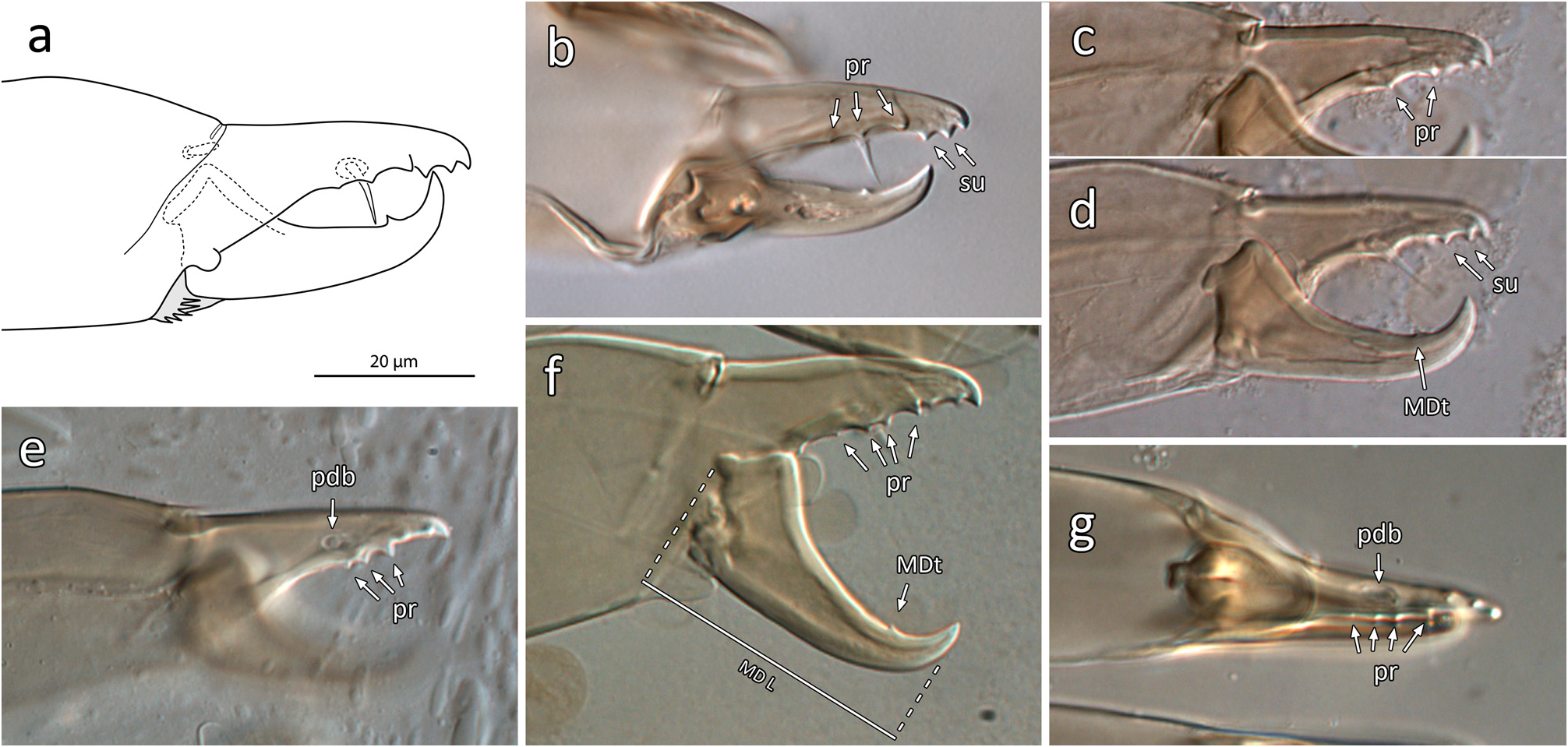
Diagnosis (adult male and female, unless stated). Dorsal shield smooth except light anterolateral lineation-reticulation, limited in female, more extensive in male; shield suboval, relatively broad (shield 1.66–1.90x as long as wide in female, 1.48–1.66x in male), with setae Z1 and S2 inserted 24–33 and 11–19 µm distant from shield margin in female; most dorsal setae relatively short ( 18–29 in female), slender, s4 (26–34) and S2 (25–37) longer, Z4 and Z5 longest (35–45 and 48–66 in female, respectively), with few barbs; j1 bases separated by distance ( 15–22 in female) similar to j1 length; gland openings gd1, gd4, gd6, gd9 visible on dorsal shield; gd1 aligned with j3–z2; gd4 directly posterior to s4, enlarged, subtriangular, opening paraxially in male, small, punctiform in female; gd9 near, anteromesal (or mesal) to seta S5. Sternal shield smooth. Ventrianal shield with three pairs of pre-anal setae ( JV1–2, ZV2) in female, four pairs (including ZV1) in male; in female, shield ornamented by transverse and oblique lineae, including three faint lineae anteriad of setae JV2, stronger lineae (ridges) and variable cells posterior to JV2, lineae weak lateral to anus; shield with truncate anterolateral corners, without constriction at level of JV2. Female with four setae ( ZV1, ZV3, JV4–5) on soft opisthogastric cuticle, male only with JV5. Gland openings gv3 small, mesal to and slightly posterior to level of JV2, with gv3–gv3 distance 0.30–0.44 x JV2–JV2 distance in female (0.32–0.51 x in male). Postanal seta ( 17–23 in female) approximately as long as para-anal setae (15–21), shorter than three pairs of pre-anal setae ( 24–31 in female). Movable cheliceral digit of female with a single, small tooth, and fixed digit with two subapical (offset) teeth followed by 2–4 teeth (rarely 5). Spermatodactyl strongly T-shaped. Calyx of spermathecal apparatus elongate, narrowly subconical, 17–25 long, 5–11 wide distally, usually slightly bent, making one side convex and the other side straighter to concave; atrium 4–6 long x 3–4.5 wide, about as wide as or slightly broader than calyx base, deeply forked at junction with major duct, thick-walled; atrium length / calyx length = 0.18–0.33; atrium and major duct of similar width. Leg IV with a single macroseta on basitarsus ( pd3), 58–74 in female.
Material examined. See Table 1. All material determined as N. barkeri by us or previous authors (slide lots #1–18), including N. barkeri syntypes (lot #7; now lectotype and paralectotype), as well as type material of N. mckenziei , N. picketti , and N. oahuensis (lots # 9–11) are included in this description. Sets of measurements (47 characters) based on different populations, including the type material of N. barkeri and its synonyms, are each presented separately (Table 2).
Redescription. Female (n=53). Dorsal idiosoma ( Figs 9a, c View FIGURE 9 , 10a View FIGURES 10–11 , 12 View FIGURES 12–15 ; Table 2). Dorsal shield 350–398 long (330–370 from j1–J5) x 198–236 wide (near S2 level; 182–220 at s4 level), suboval, margins concave at level of R1; shield essentially smooth except for a few lineae near the anterolateral margins, covering the marginal area from lateral to j1 to area lateral to s4, sometimes discernible to lateral to Z1; shield with 17 pairs of relatively short, mostly smooth setae: j1 (18–23), j3 (22–30), j4–5 (17–23), j6 (18–25), J2 (21–31), J5 (12–15), z2 (20–26), z4 (20– 28), z5 (18–23), Z1 (22–32), s4 (26–34), S2 (25–37), S4 (24–36), S5 (20–34); Z4 (35–45) and Z5 (48–66) moderately long, smooth or weakly barbed with 0–2 and 3–7 discernible barbs, respectively. Setae r3 and R1 (19– 28) relatively short, on soft cuticle lateral to shield. A total of 16 pairs of poroids and four pairs of gland pores ( gd1, gd4, gd6, gd9) visible on shield (pores gd2, gd5 and gd8 absent or not discernible); poroid idm1 slightly to obviously anterolaterad of gland pore gd6 and seta Z1 alignment (occasionally idm1 is aligned with, positioned in between gd6-Z1); one poroid ( idR3; =Rp sensu Lindquist & Evans 1965), on soft cuticle at a level anteriad of S4 ( Fig. 9c View FIGURE 9 ). Peritrematal shield fused to dorsal shield at level anteriad of j3, close to j1; peritremes narrow (4–6 wide), extending forward to or near bases of j1.
Ventral idiosoma ( Figs 9b, d View FIGURE 9 , 10b View FIGURES 10–11 , 13–15 View FIGURES 12–15 ; Table 2). Tritosternum with plumose laciniae (reaching setae h2–3 if straight), 72–77 long including a fused proximal section 33–35 long (laciniae delimited from columnar base (13– 14 long) by a faint transverse line). Sternal shield 67–76 wide (at level of coxae II), essentially smooth except a few lineae along lateral and anterior margins, usually including a pair of oblique lineae each crossing poroid iv1, meeting medially, and from which another pair of lineae sometimes originates medially across anterior of shield ( Fig. 9b, d View FIGURE 9 ); anterior margin poorly defined, with adjacent presternal area lightly sclerotised, with a few irregular transverse lineae, cuticle lightly punctate anteromesal to setae st1; lateral margins of sternal shield acutely produced at level between st2–3, sometimes rounded or truncate; setae st1–3 (27–35) smooth, on shield; st4 (27– 34) and poroid iv3 inserted on irregularly suboval metasternal platelet. Epigynal shield widest posteriorly, narrowed at level anterior to pair of smooth setae st5 (24–30), essentially smooth, sometimes irregularly punctate; with sigillae posteromesally, arranged in inversed V pattern; poroids iv5 inserted between st5 and ZV1. Ventrianal shield 116–145 long, relatively broad anteriorly (99–120), with truncate (sometimes even concave) anterolateral corners; lateral margins almost straight from level of ZV2 to level of JV4, slightly concave at level of postanal seta, just anterior to cribrum; cribrum with 2–4 irregular rows of spicules; shield weakly ornamented, mostly by transverse lineae, including three faint lineae anteriad of setae JV2, stronger lineae and variable cells posterior to JV2, lineae weak lateral to anal opening; shield bearing three pairs of relatively long (24–31) pre-anal setae ( JV1– 2, ZV2); pair of gland openings gv3, small, moderately conspicuous, slightly crescent-shaped, moderately close to each other (average 20.5 ±2.1; 18–26), 17–21 mesal to and 5–8 posterior to level of setae JV2; postanal seta (17– 23) similar in length to para-anal setae (15–21), which are inserted level with mid-point of anal opening. Peritrematal shield a narrow band of cuticle bordering peritreme laterodorsally, bearing poroid id3, and (presumed) vestige of gland opening gd3 sometimes discernible between paired sigillae on shield margin, at level between coxae II–III; poststigmatic region of shield bearing two poroids and one gland opening, and merged with parapodal element, surrounding coxa IV posteriorly, bearing gland opening gv2. Narrow endopodal elements between coxae I–II sometimes free, but usually fused to anterolateral corners of sternal shield, bearing gland opening gvb; with a narrow endopodal strip mesal to coxa IV; exopodal shield a narrow strip lateral to coxae I–IV, narrowly joining peritrematal shield posteriorly, at level slightly posterior to stigmata, and bearing an anterior gland opening ( gd10) at level between coxae I–II ( Fig. 12 View FIGURES 12–15 , homologous to gp 1 in Lindquist & Moraza, 2009), gland opening sometimes nearby in soft cuticle on a minute sclerite. Soft opisthogastric cuticle with: four pairs of smooth setae, ZV1 (24–31), ZV3 (18–24), JV4 (22–29), and JV5 (44–60); two pairs of narrow metapodal platelets, primary (outer) platelet 24– 32 long, at level of ZV1; six pairs of poroids ( iv5, 4 ivo, ivp).
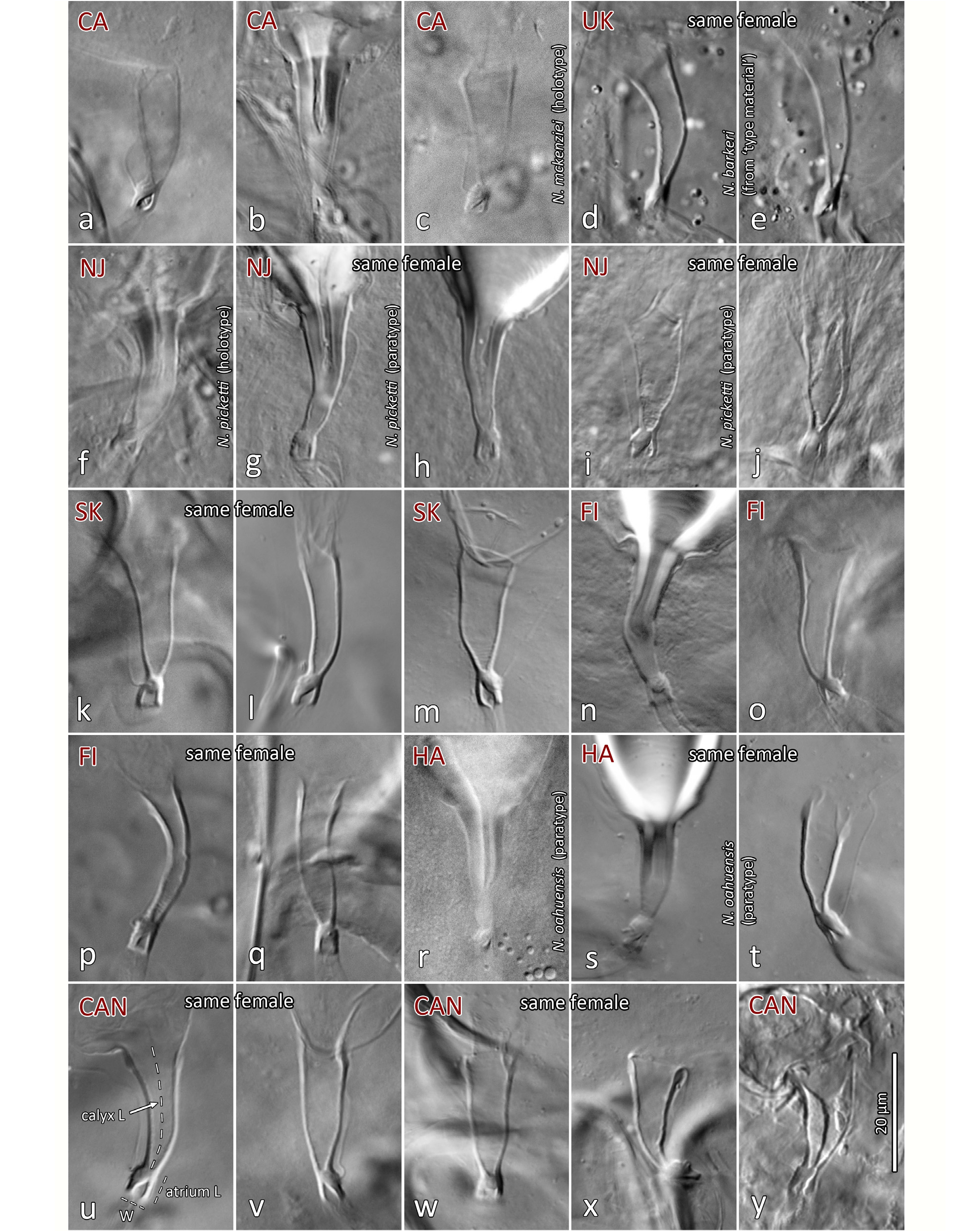
Spermatheca ( Figs 11 View FIGURES 10–11 , 17 View FIGURE 17 ; Table 2). Calyx typically narrowly elongate, cuneiform, of variable length and width, 17–25 long, 5–11 wide distally; calyx width progressively increasing from base to become parallel-sided distally or distinctly flared distally, occasionally calyx subtriangular (e.g. Fig. 17c, y View FIGURE 17 ); calyx often asymmetrical, curving to one side, with one ‘arm’ (or wall) more curved than the other (see Ragusa & Athias-Henriot, 1983: 668); calyx base slightly narrower than atrium at junction, not markedly constricted or stalked. Atrium large, 4–6 long x 3– 4.5 wide, deeply forked at junction with major duct; depending on the angle of the mount, showing a trapezoid/ subquadrate cavity ( Fig. 17g, h, j, k, n, q, w View FIGURE 17 ), or more commonly a subtriangular cavity ( Fig. 17a–f, i, l, m, o, p, r–v, x View FIGURE 17 ). The atrium is typically wider or bulging around the connection point of the minor duct, and due to this, the shape of the atrial void/cavity is dependent upon the aspect of the atrium that is in view—when the minor duct is viewed as connected to the side (e.g. Fig. 17a, f, m, o, t, v View FIGURE 17 ), the atrium cavity appears subtriangular; whereas if the atrium is viewed so that the minor duct is connected dorsally/ventrally instead (e.g. Fig. 17k, n, q View FIGURE 17 ), the bulge around the minor duct connection is no longer visible and the atrium is more symmetrical, and the atrial cavity presents as trapezoidal/ subquadrate. Minor duct very fine (ca. 0.7–1.0 µm diameter), of indeterminate length (lengths of 130–150 µm have been observed). Major duct of similar width to or slightly broader than atrium, membranous.
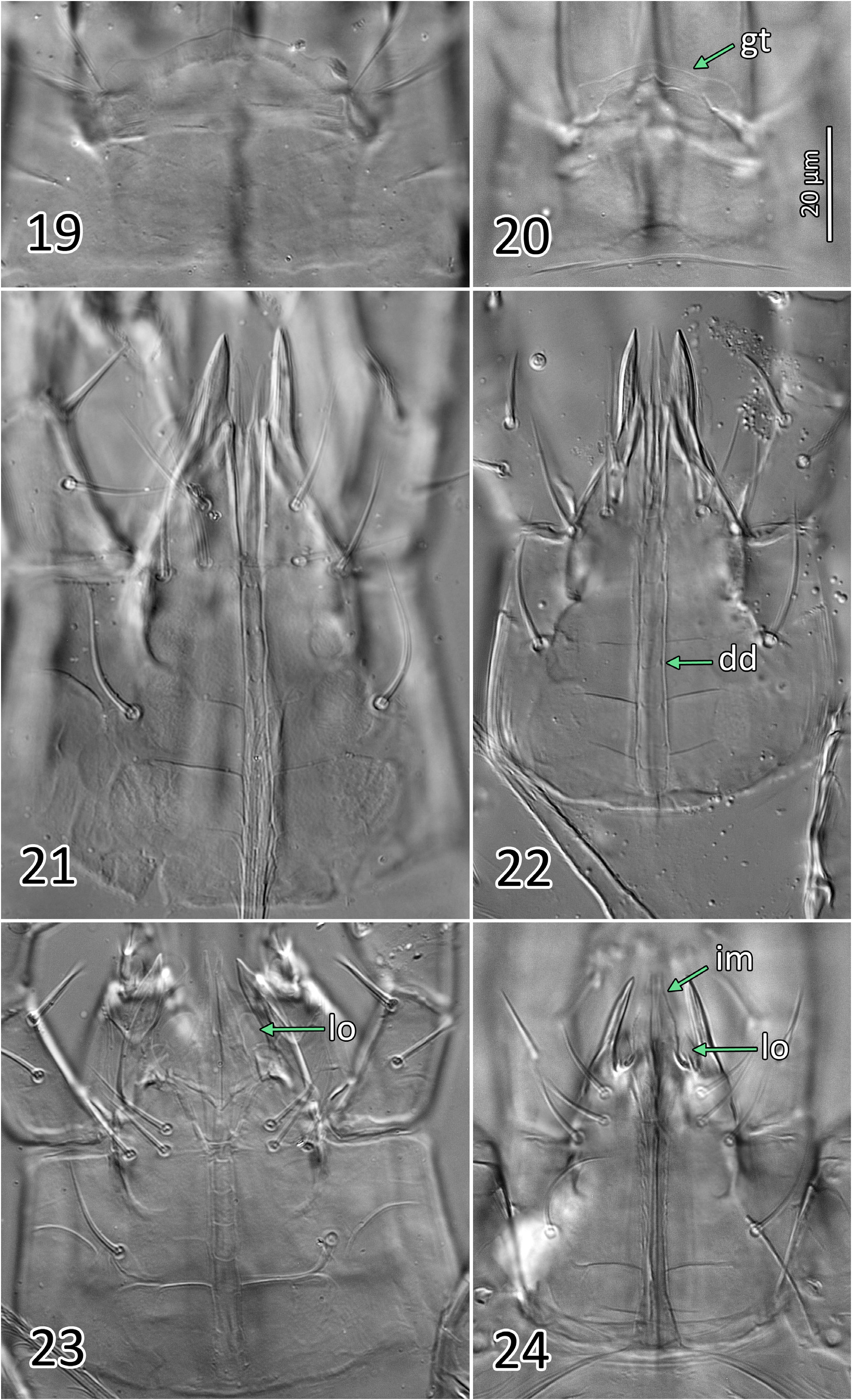
Gnathosoma ( Figs 16 View FIGURE 16 , 19, 21 View FIGURES 19–24 ; Table 2). Gnathotectum irregularly convex, smooth margins converging to form rounded apex, lateral corners typically rounded to form small bulges. Corniculi horn-like, more or less parallel to each other, and close together with bases of inner margins (level with bases of internal malae) separated by 4–7 ( Fig. 21 View FIGURES 19–24 ); entire corniculi length 33–36 (from most proximal point, visible internally). Internal malae hyaline, rounded apically, apparently without fringe, well-separated medially, flanking and clearly shorter than corniculi. Labrum broad, tapering to a blunt apex, slightly longer than (or subequal to) internal malae. Hypostomal and capitular setae smooth, h1 (23–27), h2 (22–24), h3 (20–27), pc (28–29); insertions of h2 and h3 aligned transversally. Deutosternal groove with seven rows of 2–3 denticles each, two basal rows close to each other, denticles set near lateral margins of each groove, occasionally with a third smaller denticle medially; smooth ridge anteriorly. First cheliceral segment 27–32 long, second segment including fixed digit 84–91 long, fixed digit 30–34 long from dorsal lyrifissure; fixed digit with 4–6 (rarely 7) small teeth, including (from distal to proximal) 2 subapical teeth (offset; aligned with pilus dentilis along antiaxial/outer edge of digit), and 2–4 (rarely 5) proximal teeth aligned along paraxial/inner edge of digit, with most proximal tooth at level with or slightly proximal to (setiform) pilus dentilis (distalmost proximal tooth is largest). Movable cheliceral digit 33–37 (exceptionally 39) long, with a single small tooth 7–8.5 from apex of digit; dorsal cheliceral seta short and setiform; dorsal and lateral (antiaxial) poroids (lyrifissures) present; arthrodial membrane of movable digit a simple corona. Palp chaetotaxy 2-5-6-14-15 for trochanter-femur-genu-tibia-tarsus, all setae smooth and simple except: palpgenual setae al1–al2 short, thickened and spatulate apically, palpfemoral seta al thickened, strongly spatulate; two putatively chemosensory setae on apicodorsal region of palptibia with thickened bases, appearing hollow compared to other (tactile) setae; palptarsus with an apical cluster of nine similar chemosensory setae, variously thickened basally; palp apotele 2-tined.
Legs ( Figs 25–28 View FIGURES 25–28 ). All legs with an ambulacrum, including well-developed stalk, claws and pulvillus; ambulacrum of leg IV longer (32–40) than those of legs I (17–29) and legs II–III (24–33). Chaetotaxy of leg segments I–IV matches other members of Phytoseiidae studied by Evans (1963) and Rowell & Chant (1979), except for genu II–III and tibia I bearing 7, 7 and 10 setae, respectively, which matches at least some other amblyseiines ( Rowell & Chant, 1979): coxae 2–2–2–1; trochanters 5–5–5–5 or I (1 0/3 1) (as al d / v pl), II (1 0/3 1), III (1 1/3 0), IV (1 1/3 0); femora 12–10–6–6, or I (2 3/1, 2/2 2) (as al ad / av, pd / pv pl), II (2 3/1, 2/1 1), III (1 2/1, 1/0 1), IV (1 2/1, 1/0 1), femur I–II with short al2 (6–8), ad2 (10–12), ad3 (7–9), and femur III–IV with short pd (9–11), pl (6–8); genua 10–7–7–7, or I (2 2/1, 2/1 2), II (2 2/0, 2/0 1), III (1 2/1, 2/0 1), IV (1 2/1, 2/0 1); tibiae 10–7–7–6, or I (2 2/1, 2/1 2), II (1 1/1, 2/1 1), III (1 1/1, 2/1 1), IV (1 1/1, 2/0 1); tarsi II IV 18–18–18, with ad1–pd1 reduced to inconspicuous, short (3.5–5) apical processes; tarsus I with 36 long, tactile setae, and an apicodorsal cluster of short, blunt, chemosensory setae, with two more conspicuous setae near the apical end (one spatulate in its apical half, and the other curving dorsad). All leg setae simple, slender, moderately long (14–33; except short femoral setae mentioned above), with longest setae on genu I and tibia I (ventrals and laterals), genu and tibia IV, and tarsi, especially tarsus I (tactile setae 24–39), and tarsus IV: ad2, pd2 (37–43) and pd3 (58–74; referred to as a macroseta in literature). Setae ad1 on genu IV (21–31) and tibia IV (20–25) not conspicuously longer or thicker than surrounding setae (and are therefore not considered here to be macrosetae); ventral setae of telotarsi II–IV, generally slightly thicker than other setae; ad3 and pd3 of basitarsus IV also thicker. Coxa I bearing two gland pores ( gc) ventrally at their bases, each connected to several glands with internal calyces (see Alberti & Coons, 1999: 715).
Male (n=17). Similar in chaetotaxy, adenotaxy and poroidotaxy to female except as indicated below. Idiosomal setae are 67–87% length of those of female (average across males / average across females) except JV5 (51% of female) (Table 2; as a comparative reference: male dorsal shield is on average 77% the length of female dorsal shield).
Dorsal idiosoma ( Figs 3a, b View FIGURE 3 , 5, 8 View FIGURES 4–8 ; Table 2). Dorsal shield 282–301 long x 176–192 wide (at widest point, near R1– S2 level); setae r3 and R1 captured by dorsal shield; shield oval, lateral margin convex from level of setae r3 to posterior shield margin, and peritrematal shield fused to dorsal shield to level of r3; shield mostly smooth except for lineation-reticulation along the anterolateral margins from lateral to j1 to region posterior to R1, occasionally to level of S2; Z4 (29–32) and Z5 (35–39) moderately long, mostly smooth with 0–4 discernible barbs. Gland opening gd4 conspicuous, tent-shaped, somewhat triangular when flattened; poroid idm1 aligned with and positioned in between gland pore gd6 and seta Z1, or slightly anterolaterad of gd6-Z1 alignment (e.g. Fig. 5 View FIGURES 4–8 ). Peritremes usually extending forward almost to bases of j1 (rarely less so, reaching between j1 and j3).
Ventral idiosoma ( Figs 3c, d View FIGURE 3 , 6, 7 View FIGURES 4–8 ; Table 2). Sternogenital shield 120–129 long, essentially smooth except for a few lineae along lateral margins, sometimes fine punctae discernible in anterior region ( Fig. 3d View FIGURE 3 ), shield clearly widest (86–93) at level of fusion with endopodal elements between coxae II–III (or at level of (long narrow) endopodal elements between coxae I–II when those are not broken off shield; Fig. 6 View FIGURES 4–8 , left side), bearing smooth setae st1–5 (19–27) and poroids iv1–3, and iv5 (not always discernible); presternal area weakly sclerotised, indistinctly lineate, as in female; posterior margin straight, sometimes irregular (or eroded) posterolaterally. Ventrianal shield abutting sternogenital shield, with a rounded concavity in margin posterior to each coxa IV; shield relatively broad anteriorly (133–146), lateral margins more or less convex along entire length, bearing four pairs of relatively long (19–26) pre-anal setae ( JV1–2, ZV1–2), and four pairs of poroids ( iv5, 3 ivo); shield reticulate throughout; pair of gland openings gv3, small, moderately conspicuous, slightly crescent-shaped, moderately close to each other (average 16.9 ±2.7; 13–23), 13–15 µm mesal to and 5–7 posterior to setae JV2; postanal seta (14–15) subequal in length with para-anal setae (13–15), which are inserted level with or slightly anterior to midpoint along anal opening. Peritrematal, endopodal and parapodal shields similar to those of female, except that parapodal shield usually narrowly fused to ventrianal shield. Soft opisthogastric cuticle with JV5 (24–37), and three poroids ( idR3, ivo, ivp).
Gnathosoma ( Figs 4 View FIGURES 4–8 , 23 View FIGURES 19–24 ; Table 2). Similar to that of females except the following: corniculi aligned at a convergent angle, and approximately three times more distant from each other than in female, with bases of inner margin separated by 15–22; entire corniculi length 28–30 ( Fig. 23 View FIGURES 19–24 ). Internal malae more developed than in female, projections close to each other, lightly fringed laterally, more acuminate and longer than in female, reaching level of corniculi tips; a pair of distinct, rounded hyaline lobes ( Fig. 23 View FIGURES 19–24 , ‘lo’) present between each internal mala and corniculus (absent in female), half the length of the mala; labrum similar to that of female, but longer, slightly surpassing corniculi tips. Anterior hypostome (anterior to deutosternum) more contracted than that of female, so that bases of corniculi (externally) and of internal malae much closer to level of h2–3 setae, and that h1 seta much closer to h3 seta than in female. Fixed cheliceral digit 20–23 long, broad along most of its length (from lateral aspect), with a subapical hump on dorsal margin; with 3–5 small teeth, including (from distal to proximal) one large subapical tooth (offset, aligned with pilus dentilis along antiaxial edge of digit), 2–4 proximal teeth of variable size (distalmost proximal tooth is largest), aligned along paraxial edge of digit, and a setiform pilus dentilis proximal to teeth or level with most proximal tooth. Movable cheliceral digit 21–23, with a single small tooth, and T-shaped spermatodactyl, shaft 15–17, heel 15–17, toe 18–19, together (entire “foot”) 33–35.
Legs. Leg segment chaetotaxy as in female, except setae slightly shorter than respective setae of female. Macroseta on basistarsus IV (StIV) 48–52.
Remarks. Literature records indicate that N. barkeri is widespread, found on a variety of host plants, including trees, shrubs and herbaceous plants, including crops and weeds ( Hughes, 1948; Athias-Henriot, 1966; Swirskii et al., 1998; Minarro et al., 2002; de Moraes et al., 2004; Otero et al., 2005; Papadoulis et al., 2009; Rahmani et al., 2010), as well as in stored grains, house dust, soil, litter ( Hughes, 1976; Swirskii et al., 1998; Abo-Shnaf & de Moraes, 2014), and bird nests and chicken manure (as N. oahuensis, Prasad, 1968 ). It was commercially used as a biocontrol agent in greenhouses against broad mite Polyphagotarsonemus latus (Banks) (Tarsonemidae) , and secondarily against thrips and whiteflies ( Ramakers and Van Lieburg, 1982; Hansen, 1988; Ramakers, 1988; Gillespie, 1989), though it was not as effective as N. cucumeris ( Ramakers, 1988) . For this reason, the widespread commercial production of N. barkeri seems to have since ceased, although the species can still be readily collected in commercial glasshouses ( van Houten et al., 1995). Its broad geographic distribution may, in part, be the result of its commercial use and of its ability to disperse after introduction into new regions. The specimens from lemon in California, including the types of N. californicus and N. mungeri (slide lots # 1–6), are among the few records of N. barkeri in North America outside of greenhouses, and probably represent the earliest collections of the species on the continent (1952–1958), just four years after its description (from specimens collected in England; Hughes, 1948). Its collection from citrus is not surprising, given the already diverse host associations, and its previous records on citrus elsewhere, including Chile ( Ragusa & Vargas, 2002); Japan ( Ehara, 1972; both on seedlings and fruits); Morroco (slide lot #18, Table 1; putatively same specimen as in Chant, 1959: 80, under the name N. marinus ); and eastern USA (as N. picketti ( Specht, 1968) ; in a greenhouse). It can also occur in soil, litter and on weeds in citrus orchards (e.g. Hajizadeh & Nazari, 2012; Abo-Shnaf & de Moraes, 2014). Perhaps more surprising, though, is that N. barkeri has never been reported from citrus in California again (Jim McMurtry pers. comm. 2016), and our records herein (for 1952–1958; slide lots #1–6) may be the only ones, in addition to McGregor’s (1954, 1956) original records (as T. californicus and T. mungeri ). This may indicate that the true natural habitat and host range of N. barkeri is poorly understood, as many or most of its records are from managed or disturbed habitats.
There is a total of four female (including one partial) and one male syntypes of N. barkeri mounted on three slides registered at the Natural History Museum (London, NHMUK) (Anne Baker, pers. comm.). One of the female syntypes is herein designated as the lectotype of N. barkeri (female in slide lot #7, Table 1; slide code 1982.8.16.1; Figs 10, 11c View FIGURES 10–11 ) .
Neoseiulus barkeri is also the type species of the genus Neoseiulus . Interestingly, Ragusa & Athias-Henriot (1983), while defining the genus Neoseiulus (in a more strict sense than that currently used), stated that Hughes (1948) had illustrated N. barkeri with figures of an amblyseiine female and of a male with a typhlodromine podonotal (“peltidial”) chaetotaxy, perhaps implying that Hughes had illustrated the male of a species other than barkeri . However, this is not the case, and the illustration of the male is clearly amblyseiine, with the only setae missing from the illustration being r3, for the podonotal region. This is supported by our examination of a male paralectotype of N. barkeri (illustrated, Figs 3b, d View FIGURE 3 , 4b View FIGURES 4–8 ) and of males from a culture of N. barkeri (lot #16).
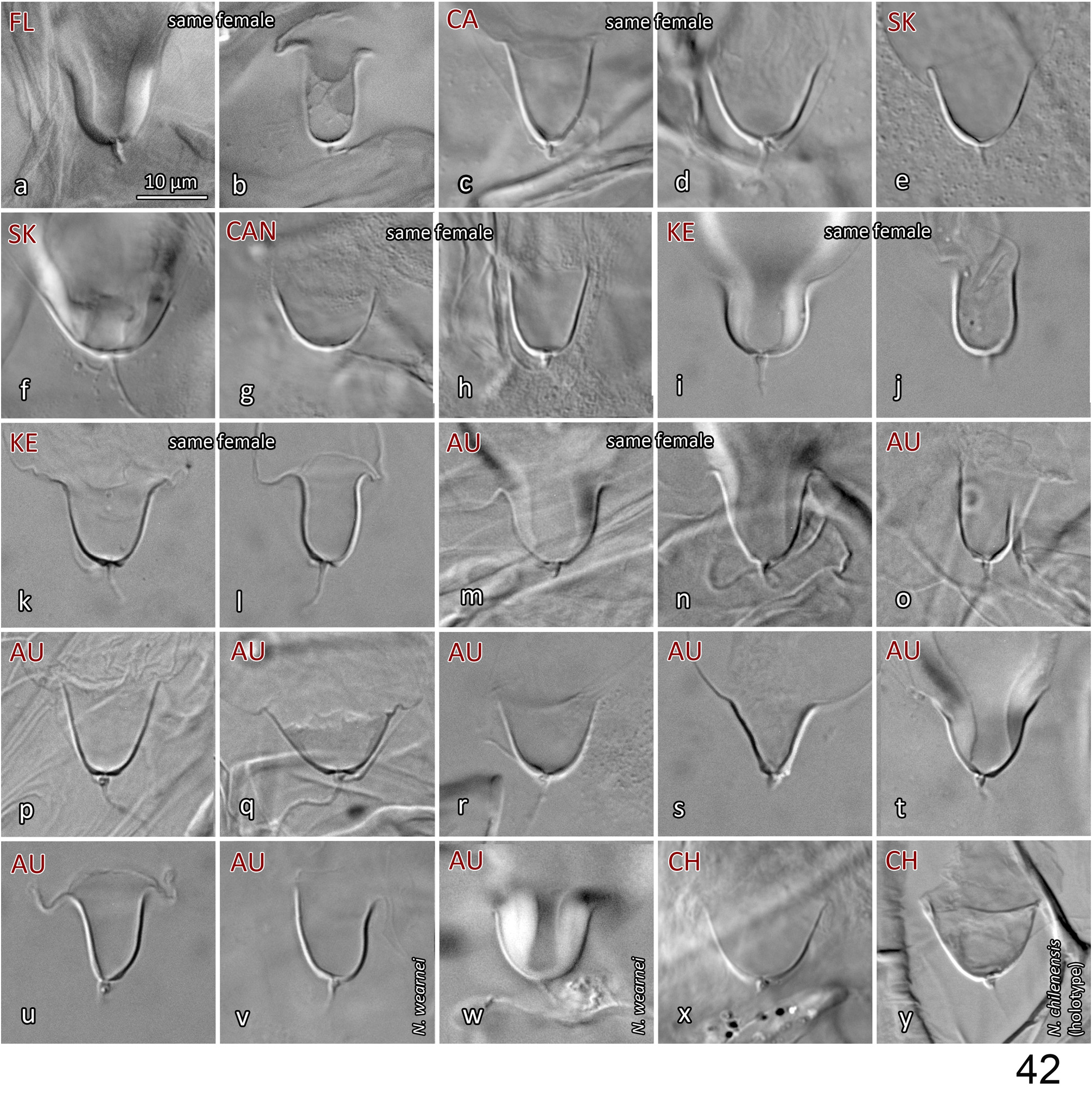
Variation in spermathecal shape. Our examination of specimens from England, USA (including Hawaii), Canada, Finland, South Korea and Morocco indicates that there is moderately strong intraspecific variation in the shape of the calyx and of the atrium of Neoseiulus barkeri . However, the range of variation present is essentially captured within each population (or sample) and most notably, can often be seen within an individual: calyx length ( Fig. 17 View FIGURE 17 g-h); calyx width (diameter) and curvature (17d-e, k-l, p-q, u-v, w-x). Additionally, some calyces appear to be more strongly constricted at the base than others, however this character state also varies within individuals ( Figs 17 View FIGURE 17 s-t, w-x). Apparent lengths of calyces can be influenced by the extent of apical sclerotisation of the walls: typically, the walls have an apical thickening that presents as a swollen ‘lip’, and ranges from strongly ( Figs 11c View FIGURES 10–11 (arrow); 17h, o, u) to poorly sclerotised ( 17t) (see also Ehara, 1972; Swirskii et al., 1998; Kolodochka, 2006). This sclerotisation is often distinct enough to be naturally included in the measured calyx length, but on other occasions it is barely discernible and hence excluded from the calyx length. Furthermore, the presence of spermatophores lodged in the calyx can obscure the delineation between calyx wall and vesicle membrane, resulting in the apparent apical extension of the sclerotisation of calyx walls ( Figs 11c View FIGURES 10–11 (right calyx, spermatophore not illustrated), 17g, h). The presence and position of the spermatophore may also influence the calyx shape ( Fig. 17g, h, n View FIGURE 17 ). Other spermathecal characters may, in part, be a function of the spermatheca’s orientation in three-dimensional space, and how the internal body fluids and structures distort it while the mite is being flattened during mounting ( Jolly et al., 2001). This is supported by the variation in the shape of the atrium in relation to the connection point of the minor duct (see Spermatheca description above; Figs 17m, n View FIGURE 17 , p-q, w-x; Papadoulis et al., 2009: 99; Beard, 2001: 75, 77, 81, 82). The variation in calyx width (at base or more distally) may also be due to the spermatheca’s orientation within the space of the body cavity, such that transverse cross-sections of the calyx would vary from circular to oval or elliptical, resulting in an apparently narrow to an apparently wide calyx. This morphological variation highlights the need to study N. barkeri populations further, on both morphological and molecular fronts, to consolidate and reinforce its species concept across the species’ entire distribution. Such research will contribute to our understanding of intraspecific versus interspecific variation, and perhaps reveal cryptic species and elucidate further synonymies. Note that a similar breadth of intraspecific variation in the shape of spermathecal calyx is also seen in N. californicus sensu Athias-Henriot ( Fig. 42 View FIGURE 42 ).
Variation is also seen in setal lengths, although values are similar across populations and ranges broadly overlap, with a few exceptions. For instance, the males and females of the N. barkeri culture from Finland (slide lot #16) show higher upper limits of lengths for some dorsal setae (e.g. j5–6, S2, S4–5); however, ranges for these setae still overlap with nearly all other samples. Another example is the N. barkeri female from ‘type material’ (lot #8): some setae (e.g. J2, z2, Z1) are shorter than most other populations, but such differences (such as the higher values for the culture from Finland) probably represent natural variation within a population, and between populations, especially given that it is from the same material as the N. barkeri lectotype (see footnote 4 under Table 1), whose morphometrics are fairly average. Our examination of the types of three previously suggested synonyms of N. barkeri , namely N. mckenziei ( holotype), N. picketti ( holotype and paratypes) and N. oahuensis ( two paratypes), indicates an overlap in morphometrics and in the shape of the spermatheca, including the N. barkeri lectotype ( Fig. 11c View FIGURES 10–11 ), thereby supporting their synonymy with N. barkeri (see further comments below). See also the similarity between the spermathecae that we studied—in particular the N. mungeri types and other females from California citrus ( Figs 11a–b View FIGURES 10–11 , 17a–b View FIGURE 17 ), and that illustrated by Ragusa and Athias-Henriot (1983) for a ‘paratype’ of N. barkeri (representing one of the three female paralectotypes, since no lectotype had been designated at that time; their Fig. 8a View FIGURES 4–8 ).
Previous descriptions. Descriptions of N. barkeri in the literature are generally compatible with our study (see references on p. 20, in paragraph on ‘spermatheca’; and Wainstein & Shcherbak, 1972; Bregetova et al., 1977: 242; Otero et al., 2005; Amano et al., 2011: 99), with a few exceptions. In particular, the spermatheca illustrated in Zannou et al. (2006) shows a calyx that is broad in its basal portion, perhaps more so than all the specimens we examined. Their measurements for the dorsal setae are also shorter than in other descriptions of N. barkeri (e.g. Ehara, 1972; Abo-Shnaf & de Moraes, 2014) and barely overlap with our measurements for the specimens we studied. The description by de Moraes et al. (1989) and Kade et al. (2011) show similarly shorter dorsal setae. The sternal shield illustrated for N. barkeri by Oliveira et al. (2012) is reticulate, suggesting that their illustrations represent a mix of two species, or a species distinct from N. barkeri . Note that at least one of the three illustrations of spermathecae in Oliveira et al. (2012) shows a calyx that is at least as distally flared as the maximum that we have observed ( Fig. 17y View FIGURE 17 ), as is the calyx illustrated by Swirski & Amitai (1985). The calyx shown in Karg (1993; the same image is reproduced in Denmark & Edland, 2002, Fig. 3P View FIGURE 3 ) appears particularly narrow, but it is however, similar in shape to that of several specimens that we have examined (see Fig. 17d, e, p, q, u View FIGURE 17 ). The description of Ryu (1997) is generally compatible with our observations for N. barkeri females except that gland openings gv3 are significantly closer to each other than we have observed (13 µm based on their illustration, vs 20.5 ± 2.1 (18–26) in our specimens). The detailed diagnostics (in the key) and illustrations of Swirski et al. (1998) appear accurate and compatible with our observations of N. barkeri , except that gland openings gv3 are atypically far apart (36 µm based on illustration) and the postanal seta is atypically longer than the para-anals and is as long as the pre-anals (based on their figure, postanal and para-anal setae are just slightly outside the ranges we observed). The few descriptions of the male of N. barkeri that include any measurements for the dorsal setae ( Ehara, 1972; Ueckerman & Loots, 1988; Oliveira et al., 2012) indicate that the dorsal setae are slightly shorter than on the male specimens that we studied, especially so for setae J2, Z4 and Z5. Note that the ventrianal shield illustrated in Nesbitt (1951) has three pairs of pre-anal setae instead of four, so it probably does not represent N. barkeri .
Sexual dimorphism. Other than McGregor (1954) (for N. californicus ), only one publication provides an illustration of the dorsal shield of the male of N. barkeri , and that is Hughes (1948) (note that the same illustration was presented in Hirschmann (1962)). Generally, male phytoseiids have a dorsal shield that resembles that of the corresponding females of the same species, but differs in several aspects. Firstly, male dorsal shields tend to be broader relative to their length and are more extensively fused with the peritrematal shield, in capturing setae r3 and R1 (although the latter can be off the shield in some males, Papadoulis et al., 2009; Chant & Yoshida-Shaul, 1989). Secondly, the pattern and expression of gland openings can differ from those of the female (e.g. gd3 hypertrophied, on dorsal shield in male N. californicus sensu Athias-Henriot vs small, on peritrematal shield in female; gd4 enlarged in male N. barkeri vs small in female; positions of idm1 and gd6 relative to seta Z1). Thirdly, shield ornamentation can also present differences, as in N. barkeri where the male dorsal shield has more reticulation in lateral regions than does the female shield, but only in the portions of the shield that are represented by soft cuticle in the female ( Figs 3b View FIGURE 3 , 5 View FIGURES 4–8 vs 9a, c). Fourthly, some setae may be shorter in the male, relatively to body size. For instance, in N. barkeri , setae JV5 of males are considerably shorter than those of females, relative to the dorsal shield ( JV5 / dorsal shield length = 9.1% ± 0.6 in males (8.2–10.2%) vs 13.9% ±0.9 (12.4–15.7%) in females); other setae of males are also shorter, such as Z5, but the contrast with females is more modest ( Z5 / dorsal shield length = 12.9% ± 0.6 in males (12.0–13.8%; n =14) vs 14.6% ± 1.1 in females (12.8–16.9%; n =26). In other cases, difference in setal length may be even more pronounced (e.g. all dorsal setae much longer in female than male of Typhlodromus carmonae Chant & Yoshida-Shaul (1983)) , and although rarely observed, setae may even be absent in one of the two sexes only (e.g. z6 present in the female but absent in the male of Paraseiulus soleiger (Ribaga) ; Chant & Yoshida-Shaul, 1989). Absence of setae in males is more typical for the opisthogastric region, where a few setae are lacking in males (e.g. ZV3, JV 4 in N. barkeri ; also ZV 1 in Nc -AH; Chant & Yoshida-Shaul, 1991). In addition, gnathosomal structures other than the chelicerae can differ between sexes, particularly the hypostome: (1) the males of N. barkeri and N. californicus sensu Athias-Henriot both have convergent, wellseparated corniculi, which are (2) flanked medially by a pair of hyaline lobes, whereas females have closely parallel corniculi and have no such lobes ( Figs 21–22 View FIGURES 19–24 vs 23–24); these lobes, present only in the male, have already been observed in N. barkeri ( Ueckermann & Loots, 1988: 149) , and in other Phytoseioidea ( Lindquist & Moraza, 2016); (3) the hypostome itself projects anteriorly to a greater extent in females than in males of both species, so that the external bases of corniculi and internal malae are well separated from h setae in females, but relatively close to h setae in the ‘contracted’ hypostome of males; (4) the internal malae of N. barkeri females are shorter, more hyaline and more rounded apically than in males, but not in N. californicus sensu Athias-Henriot in which they are identical between the sexes. Therefore, the inclusion of the male dorsal shield in descriptions, as well as the subcapitulum, in addition to other sexually dimorphic features (chelicerae; sternal and opisthogastric regions differing in the extent of sclerotisation), may provide useful diagnostic and phylogenetic information, especially given that the male has been described for only a limited number of species.
| NHMUK |
Natural History Museum, London |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Neoseiulus barkeri Hughes, 1948
| Beaulieu, Frédéric & Beard, Jennifer J. 2018 |
Neoseiulus kermanicus
| Daneshvar, H. 1987: 14 |
Amblyseius mycophilus
| Karg, W. 1970: 290 |
Amblyseius cydnodactylon
| Shehata, K. K. & Zaher, M. A. 1969: 177 |
Amblyseius oahuensis
| Prasad, V. 1968: 1518 |
Amblyseius picketti
| Specht, H. B. 1968: 681 |
Amblyseius mckenziei
| Schuster, R. O. & Pritchard, A. E. 1963: 268 |
Typhlodromus californicus
| McGregor, E. A. 1954: 89 |
Typhlodromus mungeri
| McGregor, E. A. 1954: 92 |
Neoseiulus barkeri
| Hughes, A. M. 1948: 142 |