Polydora cornuta Bosc, 1802
|
publication ID |
https://doi.org/10.5281/zenodo.170213 |
|
DOI |
https://doi.org/10.5281/zenodo.3507494 |
|
persistent identifier |
https://treatment.plazi.org/id/03EA375A-FFF8-FFB1-FECE-FB96FD65F9FD |
|
treatment provided by |
Plazi |
|
scientific name |
Polydora cornuta Bosc, 1802 |
| status |
|
Polydora cornuta Bosc, 1802 View in CoL
( Figs 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 )
Polydora cornuta Bosc, 1802: 150 View in CoL –153, p. 5, figs 7–8. Claparède, 1861: 542 –544, pl. 13, figs 12– 17. Blake and Maciolek, 1987: 11 –15, fig. la–f (Synonymy). Tena et al., 1991: 32 –35, fig. 3. Böggemann, 1998: 119, fig. 86. MacKay and Gibson, 1999: 169 –173, figs 1–6. Radashevsky and Hsieh, 2000: 205 –208, fig. 3. SatoOkoshi, 2000: 445.
Polydora ciliata: Möbius, 1873: 108 View in CoL . Fide Söderström, 1920. Surugiu and Manoleli, 1999: 24. (material reexamined). Not Johnston, 1838.
Polydora ligni Webster, 1880 View in CoL *: 119; 1886: 148–149, pl. 8, figs 45–47. Söderström, 1920: 265 –267, figs 170–174. Berkeley and Berkeley, 1936: 471 –472; 1952: 19, figs 31–33. Friedrich, 1937: 345 –347, figs 5, 6. Hartman, 1941: 309 –310; 1945: 32. Rioja, 1943: 232 –233. Mortensen, 1947: 64 –65. Hartman and Reish, 1950: 28. Hannerz, 1956: 106 –111: figs 37, 38 (larvae). Eliason, 1962: 52. Blake, 1969: 4 –10, figs 1–4 (larvae); 1971: 5–6, figs 1–2 (Synonymy); 1983: 255–256. Foster, 1971: 22 –24, figs 13–21 (Synonymy). Orensanz and Estivariz, 1971: 104 – 105, pl. IV, figs 35–37. Kudenov, 1975: 206. Rice and Reish, 1976: 285 –286, figs 1–7. Michaelis, 1978: 107 –109, 114, figs 3, 5d. Light, 1977: 70; 1978: 175–178, fig. 176. Rice, 1978: 161 – 163, figs 1–4; 1980: 182–189, figs 1, 2, 5–11, 14–16; 1981: 2–7, figs 6–8, 15, 17. Rice and Simon, 1980: 79 –115, figs 2–8, 11–13, 18, 19. Kudenov, 1982: 571 –573, fig. 1. Ramberg and Schram, 1983: 240 –242, figs 4–5. Hutchings and Turvey, 1984: 15. Johnson, 1984: 22 –24, figs 6–14. Bick and Gosselck, 1985: 236. Mustaquim, 1986: 76 –87, figs 4, 6, 9. Dauer, 1987: 42 – 44. SatoOkoshi and Okoshi, 1997: 487.
Polydora (Polydora) ligni: HartmannSchröder, 1971: 311 View in CoL –312, fig. 105d–f. Plate and Husemann, 1994: 28 (larvae).
Polydora (Polydora) cornuta: HartmannSchröder, 1996: 315 View in CoL –317, fig. 143. Polydora amarincola Hartman, 1936: 49 View in CoL , figs 6–10. ( type material reexamined). Polydora littorea Verrill, 1881: 301 View in CoL ( nomen nudum). Hartman, 1944: 336, 340, pl. 18, fig. 10, not
pl. 18, fig. 9. Fide Blake and Maciolek, 1987.
* Webster’s paper is dated 1879, but Verrill (1881: 321) reported: "Although put in type in 1879, this paper [by H.E. Webster, VIR] was not actually published until 1880”. The plates that were prepared for the paper, were published six years later ( Webster 1886), along with the original text repeated verbatim. Earlier authors all cited the former Websters paper as published in the year 1880, but Hartman (1941, 1951) and all authors after her cited it as published in the year 1879. The publication date used by earlier authors is recovered here.
Material
CANADA ATLANTIC COAST, Nova Scotia, Halifax County, Conrads Beach, 44.65° N, 63.35° W, salt marsh intertidal, sandy mud, coll. G.D. Gibson, 20 May 2001, SMF 13984 (30).
U.S.A. ATLANTIC COAST, Massachusetts, Nahant, Broad Bay, 42º26´N, 70º54´W, low tide, sand/mud tubes on muddy beach with Zostera sp., coll. F. Pleijel, 24 Aug 1997, SMF 13965 (4), USNM 177005–177010 (SEM stubs). Buzzards Bay, Wild Harbor River Marsh, coll. J. Dörjes, 29 May 1975, st. 4/6, tidal flat, SMF 10967 (29); st. 4/8, SMF 12060 (1); st. 4/9, tidal flat with Spartina sp., SMF 10972 (17); st. 3, 2 m, harbor, 2 Jun 1975, SMF 10974 (6), SMF 12061 (2). Maryland, Chesapeake Bay, 38°58' N, 76°29' W, from experimental plate, 1 m, coll. L.D. McCann and V.I. Radashevsky, 16 Sep 1998, SMF 13966 (50). Virginia, Little Gulf, bay side of Eastern shore, on oyster shells, coll. S.H. Hopkins, 8 Jul 1960, USNM 186563 (50+). South Carolina, Charleston Harbor, along shore in front of Grice Marine Laboratory at Fort Johnson, intertidal, from decaying wood among rocks with oysters, coll. J.A. Blake and N.J. Maciolek, 2 Jan 1980, USNM 98587 ( neotype), USNM 98588 (80+).
U.S.A. PACIFIC COAST, Washington, Willapa Bay, Stackpole Slough in Leadbetter Point State Park, 46°36.35´N, 124°02.59´W, low intertidal, mud between sea grass Zostera marina Linnaeus , 19‰, 2000 Exotics Expedition, 22 May 2000, SMF 13927 (5). California, Berkeley beach of San Francisco Bay, muddy intertidal, coll. O. Hartman, 10 Jul 1934, LACMNH– AHF Poly 0 628 (1 syntype of Polydora amarincola ), USNM 20214 (5 syntypes of Polydora amarincola ). Lake Merritt, Oakland, Alameda County, on tubes of the serpulid Mercierella enigmatica Fauvel , coll. G. Thorson, 8 Mar 1968, ZISP 1/ 35433 (3).
MEXICO, the Gulf of Mexico, Veracruz, Tamiahua Lagoon, st. E6, 25 °35´N, 97°35´W, 1 m, on oyster Crassostrea sp., coll. V.H. DelgadoBlas, 10 Jan 1993, ECOSUR SPIO74 (5).
BRAZIL, Espírito Santo: Anchieta, 20°40.22´S, 40°39.08´W, 3.5 m, fine sand, coll. K.G. Costa, 20 Dec 2002, MZUSP (1). Espírito Santo Bay, Vitória, 20°15´S, 40°14´W, larvae from plankton sample taken in a creek, coll. R.C. Nalesso and V.I. Radashevsky, 1 Apr 2004, 5 larvae not fixed. Rio de Janeiro: Corôa Grande, 22°54.25´S, 43°52.3´W, intertidal, coll. A. Esteves, Jun 1998, IBUFRJ 465 (4). São Paulo: Caraguatatuba, BIOTA st. 143 Pex, 23°37.6´S, 45°23.8´W, intertidal, coll. E.V. Pardo, 22 Aug 2001, MZUSP (2). Caraguatatuba, BIOTA st. 167 Pex, 23°37.5´S, 45°23.9´W, intertidal, coll. E.V. Pardo, 22 Aug 2001, MZUSP (91). Fazenda Beach, BIOTA st. 253 Pex, 23°21.4´S, 44°51.9´W, intertidal, coll. E.V. Pardo, 12 Nov 2001, MZUSP (2). Paraná, Paranaguá Bay: Pontal do Sul, mouth of Perequê tidal creek, 25°33.8´S, 48°21.4´W, intertidal, silty sand beach, coll. V.I. Radashevsky, 10 Aug 1998, on shells of the gastropod Stramonita haemastoma (Linnaeus) and bivalve Crassostrea rhizophorae (Guilding) , USNM 1020474 (22), on tube cups of the polychaete Diopatra viridis Kinberg , USNM 1020475 (40+). Ilha Rasa da Cotinga, 25°33´S, 48°25´W, intertidal, muddy sand spot in front of mangroves, coll. V.I. Radashevsky, 27 Aug 2001, SMF 13975 (2). Brasilia of Ilha do Mel, 25°33´S, 48°19´W, 0.5 m, on shell of the gastropod Pugilina morio (Linnaeus) occupied by hermit crab Clibanarius vittatus (Bosc) , coll. V.I. Radashevsky, 25 Aug 2001, SMF 13978 (1).
ARGENTINA, Buenos Aires, Puerto de Mar del Plata, coll. J.M. Orensanz, 15 Aug 1967, USNM 67577 (500+). Albufera Mar Chiquita, coll. J.M. Orensanz, 15 Jan 1970, USNM 67580 (16).
GERMANY, North Sea, Lower Saxony, German Bight (see Böggemann (1998) for station data), coll. J. Dörjes, Langeoog, 1987–1988, SMF 5376, 6202–6230 (410). Jade, st. 3a, 1967–1972, SMF 6231 (1). Carolinensiel, 13 Aug 1975, st. 1, SMF 6232 (1); st. 3, SMF 6233 (2). Banter See, Wilhelmshaven, 17 Feb 1977, st. 3, SMF 6234 (1); st. 1, SMF 6235 (2). Baltic Sea, MecklenburgVorpommern, off Poel Is., 54°01.4´N, 11°29´E, intertidal, fine sand, coll. K. Meißner, USNM 1020482 (8).
ROMANIA, Black Sea, Constanta, Agigea, 44°04.9´N, 28°38.4´E, 1 m, on limestone rock, coll. V. Surugiu, 3 Jun 1997, USNM 1020481 (8). Mangalia Lake, 43°48.8´N, 28°31.1´E, 0.2 m, mud on hard substratum, 8.95‰, coll. V. Surugiu, 9 Aug 1997, USNM 1020480 (30).
RUSSIA, Sea of Japan, Primorsky Region, coll. V.I. Radashevsky, Vostok Bay of Peter the Great Bay, 42º54.1´N, 132º43.1´E, 3 m, muddy sand bottom, on bivalve Mizuhopecten yessoensis (Jay) , 27 Sep 1994, SMF 13924 (2). Golden Horn Inlet of Peter the Great Bay, 43°06.3´N, 131°54.7´E, 1 m, in hull fouling of the R/V Akademik Alexander Nesmeyanov, 19 Nov 2004, IMBV 12386 (11); in hull fouling of the R/V Lugovoe, 3 Feb 2005, IMBV 12396 (3); in hull fouling of the R/V Professor Gagarinsky, 16 Mar 2005, IMBV 12395 (11).
KOREA, Yellow Sea, Shihwa Lake of Kyeonggi Bay (see Lee and Cha (1997) for station data), sts. 3, 4, 8–12, 14–16, N2, 37º18´–19´N, 126º37´–45´E, 2 m, muddy sand, coll. J.H. Cha, 1994, 1996, 1999, USNM 1020476–1020479 (680+), SMF 13919–13923, 13925, 13926, 13952–13955, 13968, 13969, 14003, 14013 (344); intertidal, coll. J.H. Cha and V.I. Radashevsky, Jun 2000, 100+ spec. examined alive, not preserved.
TAIWAN, Taiwan Province, Hsinchu county, Hsiangshan, 24°50´N, 120°54´E, intertidal, on tube of the polychaete Diopatra sugokai Izuka , coll. H.L. Hsieh, 28 Mar 1990, ASIZ W43 (1). Tainan County, Tsengwen River estuary, 23°04´N, 120°05´E, < 2 m, mud, coll. H.L. Hsieh, 26 May 1995: st. 4M, USNM 186413 (1); st. 4N, ASIZ W44 (1).
OFF MAINLAND CHINA, Fuchien Province, Tzu Lake of Kinmen Island, 24°28´N, 118°18´E, < 2 m, sandy or muddy bottom: coll. H.L. Hsieh, st. 12, 28 Feb 1996, ASIZ W45 (1); st. 2, 28 Feb 1996, ASIZ W46 (2); st. 3, 28 Feb 1996, ASIZ W47 (1); st. 4, 30 May 1996, ASIZ W48 (3); st. 5, 30 May 1996, ASIZ W49 (1); st. 5a, 28 Feb 1996, ASIZ W50 (9); st. 7, 28 Feb 1996, ASIZ W51 (1); st. 8, 30 May 1996, ASIZ W52 (1); st. 10, 28 Feb 1996, USNM 186414 (2); st. 4, 28 Feb 1996, USNM 186415 (5); st. 8, 28 Feb 1996, USNM 186416 (2).
Adult morphology
Up to 32 mm long and 1.5 mm wide for 90 chaetigers. Worms with body light tan in life, pigmentation reduced or totally lacking, except for fine continuous black line often present on palps, along edges of ciliated food groove. Worms with up to 60 chaetigers usually having black spots on lateral sides of chaetigers from 7–10 to 10–19. Smaller worms translucent in life, with retained black and yellow larval pigmentation as small distinct melanophores on lateral sides, from chaetigers 2–7 to 10–22, and light yellow pigment diffused on lateral lips of peristomium and anterior part of prostomium between lobes. Yellow chromatophores arranged in three rows on ventral side of chaetigers from 5– 7 to 16–21; median chromatophores larger and more intensive. Black and yellow pigment diffused on pygidium in some small worms. Black pigment usually retained after fixation but yellow one disappearing.
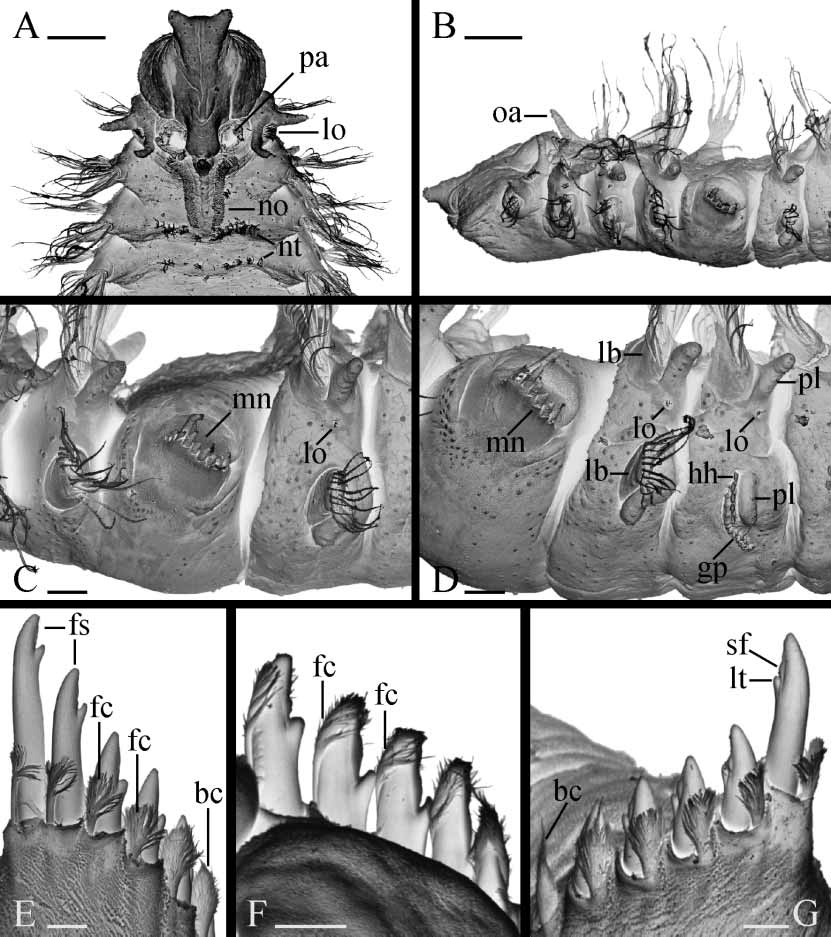
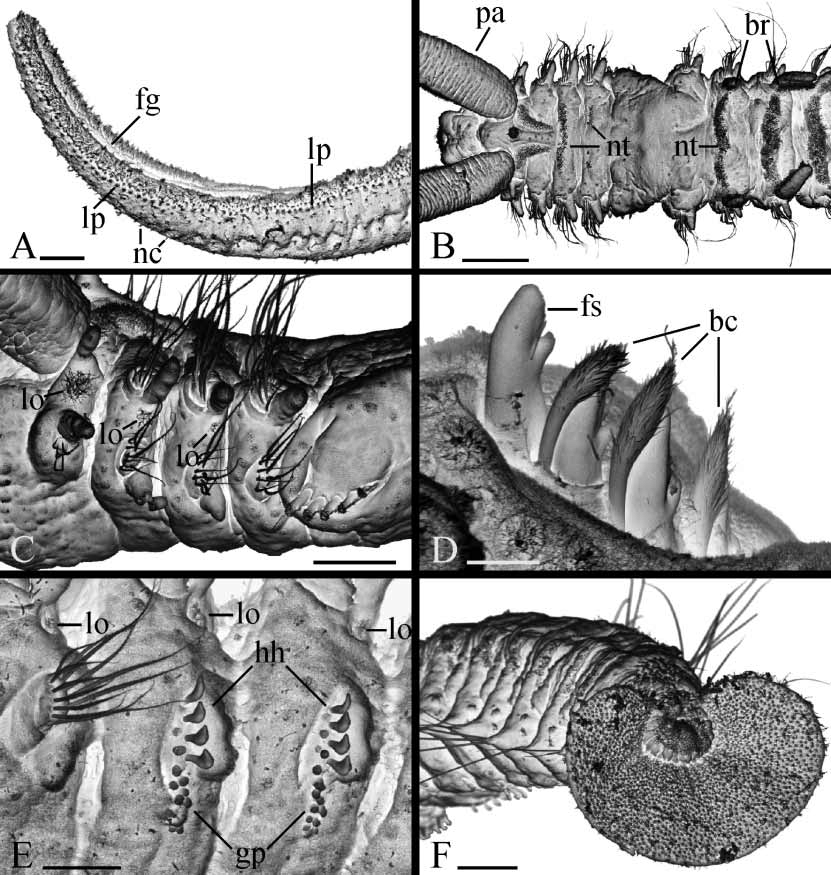
Prostomium anteriorly bifurcated and flaring laterally ( Fig. 1 View FIGURE 1 A). Two pairs of black eyes arranged trapezoidally, absent in individuals regenerating anterior segments. Eyes often reddish in fixed specimens. Low narrow caruncle extending to end of chaetiger 3, shorter in small worms. Nuchal organs as ciliated grooves on either side of caruncle. Cirriform occipital antenna on caruncle usually prominent, at level of chaetiger 1 ( Fig. 1 View FIGURE 1 B), occasionally short. Palps as long as 15–35 chaetigers, with longitudinal groove lined with fine frontal cilia, laterofrontal motile compound cilia (cirri) bordering groove, short laterofrontal papillae with nonmotile cirri arranged in two or three rows along either side of groove, and short compound nonmotile cilia arising directly from palp surface and scattered on lateral and abfrontal palp surfaces ( Fig. 2 View FIGURE 2 A).
Chaetiger 1 with well developed cirriform postchaetal lamellae in both rami; notochaetae absent; short capillaries present in neuropodia. Prechaetal lamellae absent in all parapodia. Parapodial lobes and postchaetal lamellae well developed on anterior chaetigers except chaetiger 5 ( Figs 1 View FIGURE 1 C,D, 2C), gradually reducing on posterior chaetigers. Posterior notopodia with only capillaries ( Fig. 2 View FIGURE 2 F).
Scale bars: A–B, 100 µm. C, D, 50 µm. E–G, 10 µm. A–D, U.S.A., Massachusetts. A, B, USNM 177007. C, USNM 177010. D, USNM 177006. E, F, Brazil, Paraná (USNM 1020475). G, U.S.A., Maryland (SMF 13966).
Scales: A, C, F, 50 µm. B, 100 µm. D, 10 µm. E, 30 µm. A, U.S.A., Massachusetts (USNM 177006). B–F, Brazil, Paraná. D, E, USNM 1020474. B, C, F, USNM 1020475.
Chaetiger 5 greatly enlarged, overlapping chaetiger 6 dorsally, with up to 8 major modified spines alternating with delicate companion chaetae, postchaetal lamellae lacking ( Figs 1 View FIGURE 1 C,D, 2B,C). Dorsal superior capillaries invariably absent. One or two short ventral capillaries occasionally present in 18–22chaetiger recently settled juveniles, always absent in larger individuals. Major spines and companion chaetae arranged in a slightly curved horizontal or oblique double row ( Figs 1 View FIGURE 1 C,D, 2C). Major spines falcate, with lateral tooth and narrow thin subdistal longitudinal flange or keel positioned laterally on main fang distal to lateral tooth ( Figs 1 View FIGURE 1 E–G, 2D); both lateral tooth and flange visible in unworn posterior spines, often broken or absent on worn anterior spines. Companion chaetae closely adhering to convex side of major falcate spines. One or two newly developed posterior companion chaetae often with compact, broomlike distal tip covering major spine as a cap or hood ( Fig. 1 View FIGURE 1 E,G). Older anterior companion chaetae with feathery, dishevelled tip ( Fig. 1 View FIGURE 1 E–G), under light microscope appearing bifurcated when fibres of broom bunched on one side of stem. Occasionally, both anterior and posterior companion chaetae compact, broomlike ( Fig. 2 View FIGURE 2 D).
Hooded hooks in neuropodia from chaetiger 7, up to 15 in a vertical series, not accompanied by capillaries ( Figs 1 View FIGURE 1 D, 2E). Hooks bidentate, with shaft slightly curved, having constriction on upper part.
Branchiae from chaetiger 7, nearly fullsized at first appearance or shorter on chaetiger 7 than on chaetiger 8 ( Fig. 2 View FIGURE 2 B), gradually diminishing in size on posterior part of body and absent on 4–10 posteriormost chaetigers. Branchiae flattened, with surfaces oriented parallel to body axis, free from notopodial postchaetal lamellae.
Small nototrochs with short cilia on chaetiger 3 and also often on chaetiger 4, always absent on chaetigers 5 and 6; nototrochs with longer cilia from chaetiger 7 onwards. All nototrochs composed by single row of cilia both in females and males ( Figs 1 View FIGURE 1 A, 2B). Pygidium flaring cup to disc with distinct middorsal gap to narrow incision, white in color in live and in fixed specimens due to numerous spindleshaped, glandular epithelial cells. On SEM images, separate glandular cells extending beyond pygidial surface with short filaments of hardened secretion protruding from distal end ( Fig. 2 View FIGURE 2 F).
Lateral ciliated organs as small pits with nonmotile cilia between noto and neuropodia on all chaetigers but 4 and 5 ( Figs 1 View FIGURE 1 C,D, 2C,E). Lateral organs on chaetiger 1 largest, with 2–4 parallel, closely situated rows of cilia ( Fig. 1 View FIGURE 1 A,B); organs rounded on following chaetigers.
Glandular pouches from chaetiger 7, diminishing in size after chaetiger 11 or 12, single throughout. Large, flaskshaped secretory cells of pouches opening to exterior individually and appearing externally as small papillae below and in front of vertical row of hooded hooks ( Figs 1 View FIGURE 1 D, 2E).
Gizzardlike structure absent in digestive tract.
Metanephridial segmental organs from chaetiger 7, opening to exterior laterally on anterior, sterile segments and dorsally on gametogenic segments; consegmental organs opening separately throughout. Distal part of segmental organs in female gametogenic segments inflated, whitish in life, usually taking up intensely methyl green stain in fixed specimens. Middle part of segmental organs in male gametogenic segments expanded, formed by large urnshaped cells, iridescent in reflecting light in life. First gametogenic segment with nonmodified excretory segmental organs both in females and males.
Reproduction
Polydora cornuta is gonochoristic. Similar numbers of each sex were observed in a population from Paranaguá Bay, Brazil ( 10 females, 11 males in 21 mature individuals). Gametes developed along segmental blood vessels in middle chaetigers, from chaetiger 13–15 to chaetiger 15–33. Larger worms had a greater total number of gametogenic segments.
In males, testis contained only spermatogonia; diads and tetrads of primary and secondary spermatocytes, octads of spermatids, and separate spermatozoa floated freely together in the coelomic cavity. Spermatozoa were introsperm with elongated straight head about 1 µm in diameter, head+middlepiece about 15 µm long, acrosome 1.5 µm, nucleus 7.5 µm, middlepiece 5.5 µm, and flagellum 44 µm.
Brooding females were collected in Paranaguá Bay in August 1998 and August 2001. Paired intraepithelial seminal receptacles were present on the dorsal side of gametogenic segments, posterior to the nototrochs, medial to the base of the branchia. The receptacles were oval or rounded and contained clusters of inactive spermatozoa. In each female, ovaries contained previtellogenic and vitellogenic oocytes of various diameter. Large oocytes occurred freely in the coelomic cavity. Females deposited eggs in capsules which were joined to each other in a string or sometimes loosely held together. Each egg capsule was attached by two thin stalks to the inside wall of the tube and contained up to 30 eggs ( Fig. 3 View FIGURE 3 A). Three females produced 11, 16, and 18 capsules respectively with about 300, 450, and 500 eggs per string. The eggs were spherical, 100–110 µm in diameter, filled up with yolky globules. The majority of eggs in broods developed synchronously into larvae, but a few eggs in each capsule were abortive. When no yolky globules were left in the digestive tract, the 3chaetiger larvae about 260 µm long hatched, escaped the mother’s tube and entered the plankton. Females brooding larvae in capsules had next generation of vitellogenic oocytes developing in the ovaries.
Larval morphology
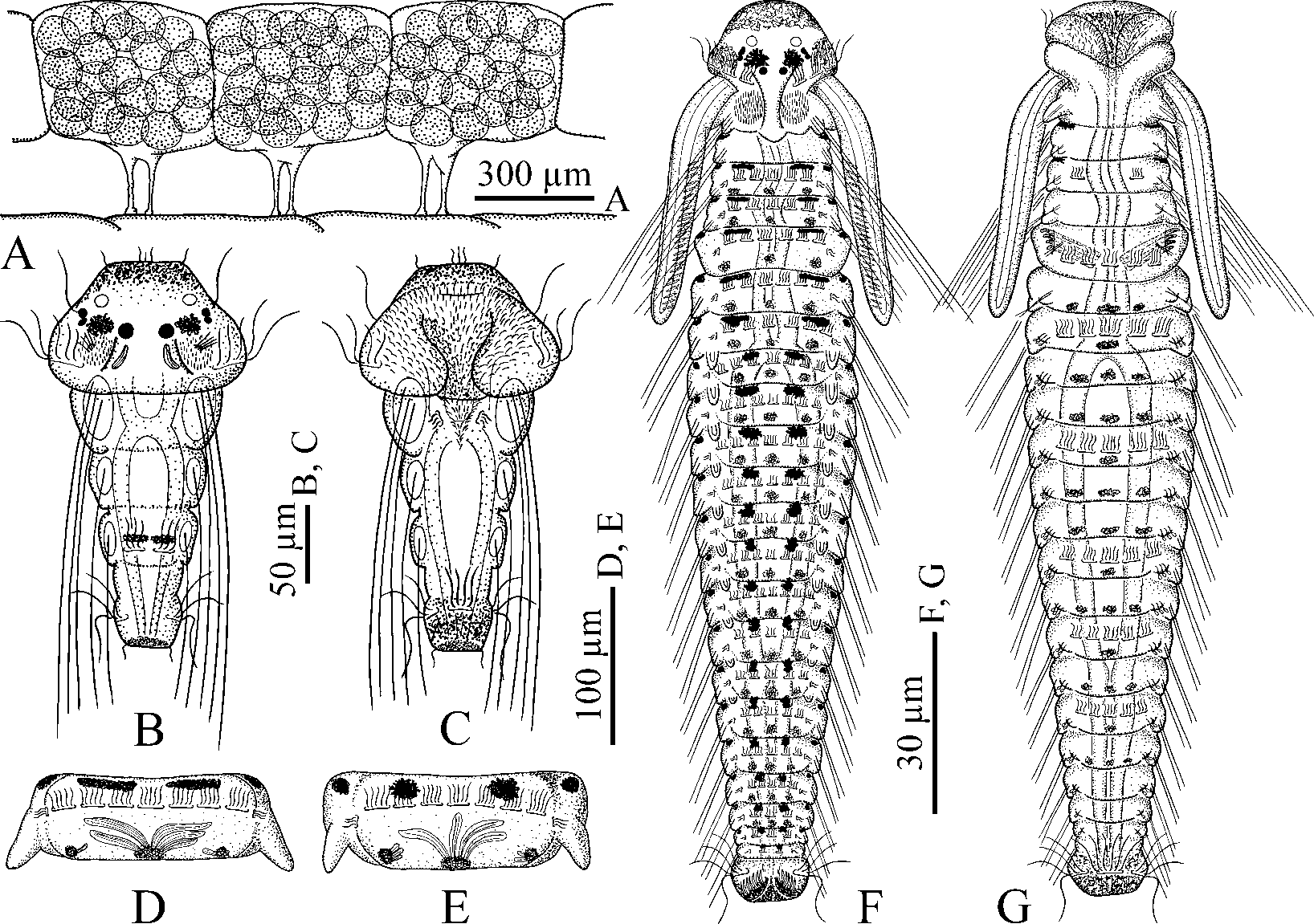
Threechaetiger larval morphology. Hatched threechaetiger larvae ( Fig. 3 View FIGURE 3 B,C) with yellow pigment diffused on anterior end of prostomium, ventral and lateral sides of pygidium, one pair of indistinct melanophores on dorsal side of chaetiger 3, and black pigment diffused on distal end of pygidium. Three pairs of black eyes present, comprised of two pairs of lateral eyes and one pair of median eyes, with lateral eyes on either side situated close to one another and therefore appearing as one pair of eyes. Ramified melanophores present between lateral and median eyes. One pair of spherical, transparent unpigmented ocelli in anterior part of prostomium, in front of eyes. Nuchal organs as rounded ciliated patches in depressions on dorsolateral sides of prostomium, in front of prototroch. Small boss with fine nonmotile cilia in ciliated patch of each nuchal organ. One pair of long motile compound cilia near lateral eyes. One pair of intraepithelial bananashaped glandular cells with striated content posterior to median eyes on posterior, slightly elevated part of prostomium.
Chaetae as long larval bristles in notopodia; those of chaetiger 1 longest, extending posteriorly beyond pygidium. Bristles slightly curved, with fine serrations on convex side. Prototroch and telotroch well developed. Nototroch and one pair of grasping cilia on sides of nototroch on chaetiger 3. Gastrotrochs absent. Short triangular neurotroch on chaetiger 1; one pair of small companion ciliated cells on sides of neurotroch. Ventral ciliated pit lacking.
Lateral lips of peristomium forming voluminous vestibulum covered by numerous, actively beating, short cilia. Long compound, probably sensory cilia arranged along inner sides of peristomial lips and between ciliated cells of prototroch. Vestibulum leading into short oesophagus separated from midgut by muscular sphincter in chaetiger 1. Midgut with wide lumen, without yolky globules in wall. One pair of protonephridia in chaetiger 1.
Growth to late larvae. Between 3 to 18–25 chaetigers larvae are swimming actively and feeding on the plankton. As growth proceeds, new segments develop successively one by one in the growth zone in front of pygidium, black and yellow pigmentation appear on chaetigers. Palps develop on posterolateral sides of peristomium, behind prototroch. The middorsal part of prostomium elongates posteriorly as a narrow caruncle, and ciliated patches of nuchal organs shift their position from prostomium to chaetiger 1. Short winged capillaries appear in neuropodia from chaetiger 1 to 6, and in notopodia among long larval bristles on all chaetigers but 1 and 5. Neuropodial hooks and branchiae develop on chaetiger 7 and successive chaetigers. Larval bristles do not develop in neuropodia and short capillaries do not appear in hookbearing neuropodia. Modified chaetae develop in notopodia on chaetiger 5 among larval bristles. Esophagus elongates, thus midgut shifts along mesenteries into more posterior chaetigers. Glandular pouches develop from chaetiger 6 onwards but later reduce in chaetiger 6 and become discernable only from chaetiger 7. Second pair of protonephridia develop in chaetiger 2 and then metanephridia appear in chaetiger 7 and successive chaetigers. Larvae 1200–1300 µm long for 17–18 chaetigers are ready for settlement and metamorphosis. Largest larva caught in the plankton was about 1700 µm long for 25 chaetigers.
20–22chaetiger pelagic larvae ( Fig. 3 View FIGURE 3 F,G). Late larvae 1400–1500 µm long, with yellow pigment diffused in anterior part of prostomium, lateral lips of peristomium and on ventral and lateral sides of pygidium, and large yellow chromatophores on ventral side from chaetigers 5–7 to 17–19. Usually single midventral chromatophores on gastrotrochbearing chaetigers, and three chromatophores arranged in transverse line on other chaetigers. One pair of large ramified melanophores between median and lateral eyes. Melanophores on peristomial lips lacking. Dorsal paired melanophores from chaetiger 3 onwards, situated along anterior edge of chaetigers; melanophores on anterior chaetigers transversally elongated, with those from chaetiger 8 ramified, stellar, often extending onto posterior edge of preceding chaetiger, and melanophores on chaetiger 7 of intermediate shape. Dorsolateral melanophores absent on chaetigers. Lateral melanophores from chaetiger 2 onwards, on anterolateral edges of chaetigers; those on chaetigers 2 and 3 largest, extending onto ventrolateral sides. Small melanophores scattered on ventral side of chaetigers among yellow chromatophores. Small vesiculate melanophores with diffused granules of melanin from chaetiger 3 or 4 onwards, situated along posterior edge of chaetigers middorsally and dorsolaterally. Vesiculated appearance of melanophores due to associated large, elongated, transparent glandular cells with striated contents, up to 45 µm long and 8 µm in diameter, opening to the exterior individually on middle and dorsolateral sides of chaetigers. Usually two to four glandular cells present middorsally and one to three smaller cells present on dorsolateral sides of chaetigers ( Fig. 3 View FIGURE 3 D,E).
Prostomium rounded anteriorly, extending posteriorly as a narrow caruncle to end of chaetiger 1. Three pairs of black eyes; lateral eyes usually obscured by large ramified melanophores. One pair of transparent unpigmented ocelli 13–15 µm in diameter in front of eyes. Nuchal organs as oval ciliated fields on either side of caruncle on chaetiger 1. Occipital antenna not yet developed. Palps as long as 4–7 chaetigers, with longitudinal ciliated groove, and fusiform cells regularly arranged on sides of groove in distal half of each palp.
Prototroch composed by two transverse ciliary bands positioned on lateral sides of head. Metatroch composed by two shorter transverse ciliary bands positioned on ventral sides of lateral peristomial lips, posterior to level of prototroch, often reduced at this stage. Nototrochs, each composed of 5–7 ciliated cells, and grasping cilia from chaetiger 3 onwards. Short triangular neurotroch extending posteriorly over ventral peristomial lip. One pair of small companion ciliated cells on sides of neurotroch, with both neurotroch and ciliated cells often reduced in big larvae. Ventral ciliated pit lacking. Gastrotroch on chaetiger 3 composed of two small cells bearing short cilia, then on chaetigers 5, 7, 10, 13, 15 and 17 composed of 5 or 7 large cells with longer cilia. Telotroch interrupted middorsally.
Up to ten long larval serrated bristles in all notopodia but chaetiger 5; bristles lacking in neuropodia. Adult winged capillary chaetae in all notopodia but chaetigers 1 and 5, and in neuropodia of chaetigers 1–6. Hooded hooks in neuropodia from chaetiger 7, up to 5 in a series, with bidentate tips, and slightly curved shaft having constriction on upper part ( Fig. 4 View FIGURE 4 A). Hooks not accompanied by any capillaries.
Chaetiger 5 larger than 4 or 6, with 4 or 5 heavy falcate spines, an equal number of closely adjoined companion chaetae ( Fig. 4 View FIGURE 4 C–G), and two short, slender ventral capillaries ( Fig. 4 View FIGURE 4 H); long larval serrated bristles, dorsal superior capillaries and postchaetal lamellae lacking. Falcate spines as in adults except one larva (of about 20 examined from the plankton) had a spine with two lateral teeth on opposite sides but no subterminal flange ( Fig. 4 View FIGURE 4 B). The first companion chaeta slender, with thin distal end curved sigmoidaly ( Fig. 4 View FIGURE 4 C). Successive companion chaetae with straight dishevelled, feathery distal end ( Fig. 4 View FIGURE 4 E– G).
Branchiae from chaetiger 7 to 12–16.
Pygidium a small, rounded cup, with distinct dorsal incision and numerous fusiform glandular cells.
Lateral organs between noto and neuropodia on all chaetigers as pits about 5 µm in diameter with nonmotile cilia 15–20 µm long.
Glandular pouches in neuropodia from chaetiger 7, large in chaetigers 7–9 and gradually diminishing in following chaetigers.
bidentate hooded hook from neuropodium of chaetiger 7 of a 22chaetiger larva. B, abnormal falcate spine with two lateral teeth, of chaetiger 5 of a 20chaetiger larva. C–G, a set of modified notochaetae of chaetiger 5 of a 22chaetiger larva, left side chaetae in ventral view. C, provisional companion chaeta with sigmoid distal end. D, oldest anterior falcate spine with lateral tooth and subterminal longitudinal flange. E–G, co mpa ni on chaetae with dishevelled, feathery distal end closely adjoined to heavy falcate spines. H, ventral capillary from neuropodium of chaetiger 5. I, recently settled 20chaetiger juvenile, anterior end, dorsal view, showing remains of larval yellow pigment on prostomium and peristomium, and black pigment on dorsal and lateral sides of chaetigers. J, a 40chaetiger female, anterior end, dorsal view, showing remains of larval yellow pigment on prostomium and peristomium, and black pigment on lateral sides of chaetigers.
Voluminous vestibulum leading into oesophagus which extending through end of chaetiger 7. Ventral buccal bulb absent. Midgut wall with numerous lipid globules. Blood system well developed and functional but blood colorless. Main dorsal vessel bifurcated in head into circumoesophageal vessels. Single palpal blood vessels arising from anterodorsal part of each circumoesophageal vessel and extending through tip in each palp. Circumoesophageal vessels joining midventrally in chaetiger 2 forming main ventral vessel.
Two pairs of protonephridia in chaetigers 1 and 2. Metanephridia from chaetiger 7.
Settlement and metamorphosis
Larvae of P. cornuta settled readily in the laboratory. Smallest juvenile was about 1200 µm long for 18 chaetigers. Settlement was accompanied by gradual metamorphosis and loss of provisional larval features, e.g., all trochs except nototrochs, long serrated bristles, and chaetiger 5 companion chaetae with sigmoid distal end. Unpigmented ocelli and protonephridia were no longer visible. Larval ciliated patches of nuchal organs were replaced with adult ciliary bands. Switch to a new mode of feeding after settlement was enabled by rapid elongation of the palps, modification of the prostomium, enlargement of the ventral peristomial lip and transformation of the lateral peristomial lips into dorsolateral ciliary folds. Newly settled individuals built small tubes of silty particles agglutinated with the mucus probably secreted by glandular cells on dorsal side of chaetigers. After settlement, dorsal melanophores were reduced but lateral melanophores and yellow pigment remained for longer period; occipital antenna developed on the prostomium, and one of lateral eye pairs was reduced, or, most probably, lateral eyes were fused and therefore appeared as one pair of eyes ( Fig. 4 View FIGURE 4 I).
In further development, prostomium became bifurcated anteriorly, caruncle and nuchal ciliated bands elongated posteriorly until the end chaetiger 3, nototrochs were lost on chaetigers 5, 6 and occasionally on chaetiger 4, capillary neurochaetae were lost on chaetiger 5, and lateral organs reduced on chaetigers 4 and 5 ( Fig. 4 View FIGURE 4 J). Worms developed gametes one or two weeks after settlement.
Smallest male was about 2 mm long for 22 chaetigers. It matured in the laboratory one week after settlement and had spermatozoa in chaetigers 13 and 14, and spermatocytes in chaetiger 15. Two weeks after settlement, largest male was 4 mm long for 31 chaetigers with spermatozoa in chaetigers 14–19.
Smallest females with 30 and 31 chaetigers had oocytes up to 24 µm in diameter in one week after settlement in the laboratory. Two weeks after settlement, largest female was 5.5 mm long for 37 chaetigers with oocytes up to 100 µm in diameter in chaetigers 14–24. Females of similar size with oocytes of similar diameter were also collected in Paranaguá Bay.
Distribution
Polydora cornuta has earlier been reported from the East, West and Gulf coasts of North America, Caribbean Sea, Argentina, northern and southern Europe, Australia, China, Korea, Japan, and India, and thus appears distributed in temperate and subtropical zones worldwide. The species is here recorded for the first time for Brazil, the Gulf coast of Mexico, and the Pacific coast of Russia.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Polydora cornuta Bosc, 1802
| Radashevsky, Vasily I. 2005 |
Polydora (Polydora) cornuta : HartmannSchröder, 1996 : 315
| Hartmann-Schroder 1996: 315 |
| Hartman 1944: 336 |
| Hartman 1936: 49 |
| Verrill 1881: 301 |
Polydora (Polydora) ligni : HartmannSchröder, 1971 : 311
| Plate 1994: 28 |
| Hartmann-Schroder 1971: 311 |
Polydora ligni
| Sato-Okoshi 1997: 487 |
| Dauer 1987: 42 |
| Mustaquim 1986: 76 |
| Bick 1985: 236 |
| Hutchings 1984: 15 |
| Johnson 1984: 22 |
| Ramberg 1983: 240 |
| Kudenov 1982: 571 |
| Rice 1980: 79 |
| Michaelis 1978: 107 |
| Rice 1978: 161 |
| Light 1977: 70 |
| Rice 1976: 285 |
| Kudenov 1975: 206 |
| Foster 1971: 22 |
| Orensanz 1971: 104 |
| Blake 1969: 4 |
| Eliason 1962: 52 |
| Hannerz 1956: 106 |
| Hartman 1950: 28 |
| Mortensen 1947: 64 |
| Rioja 1943: 232 |
| Hartman 1941: 309 |
| Friedrich 1937: 345 |
| Berkeley 1936: 471 |
| Soderstrom 1920: 265 |
Polydora ciliata : Möbius, 1873 : 108
| Surugiu 1999: 24 |
| Mobius 1873: 108 |
Polydora cornuta
| Radashevsky 2000: 205 |
| Sato-Okoshi 2000: 445 |
| MacKay 1999: 169 |
| Boggemann 1998: 119 |
| Tena 1991: 32 |
| Blake 1987: 11 |
| Claparede 1861: 542 |
| Bosc 1802: 150 |