Lissodendoryx ( Ectyodoryx ) diegoramirezensis, Fernandez, Julio C. C., Cárdenas, César A., Bravo, Alejandro, Lôbo-Hajdu, Gisele, Willenz, Philippe & Hajdu, Eduardo, 2016
|
publication ID |
https://doi.org/10.11646/zootaxa.4092.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:51F44763-E817-4E58-A4AC-525E63B6D27B |
|
DOI |
https://doi.org/10.5281/zenodo.5615611 |
|
persistent identifier |
https://treatment.plazi.org/id/03EF87BE-FFC0-FFB2-419B-FDD1FA2574CA |
|
treatment provided by |
Plazi |
|
scientific name |
Lissodendoryx ( Ectyodoryx ) diegoramirezensis |
| status |
sp. nov. |
Lissodendoryx ( Ectyodoryx) diegoramirezensis View in CoL sp. nov.
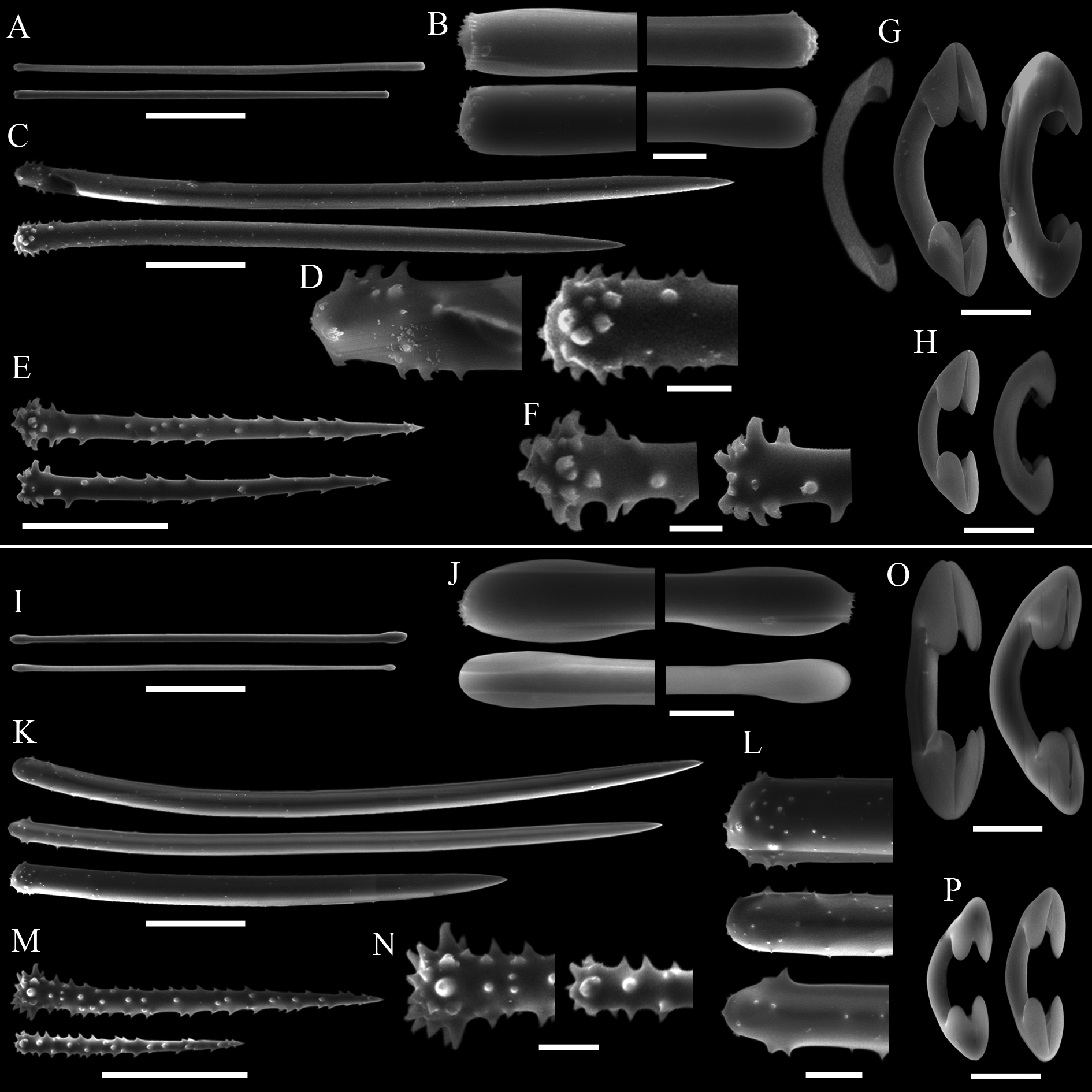
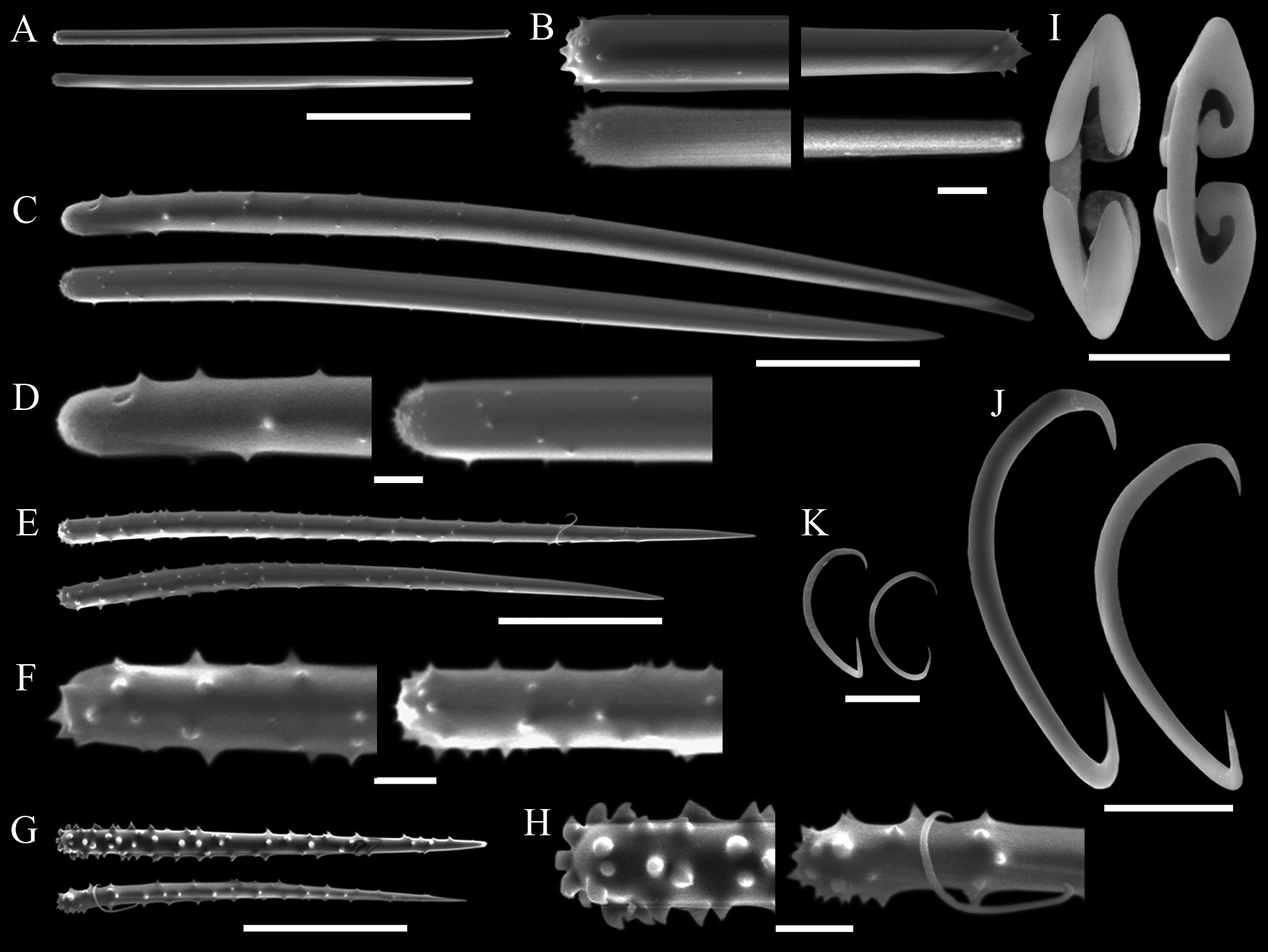
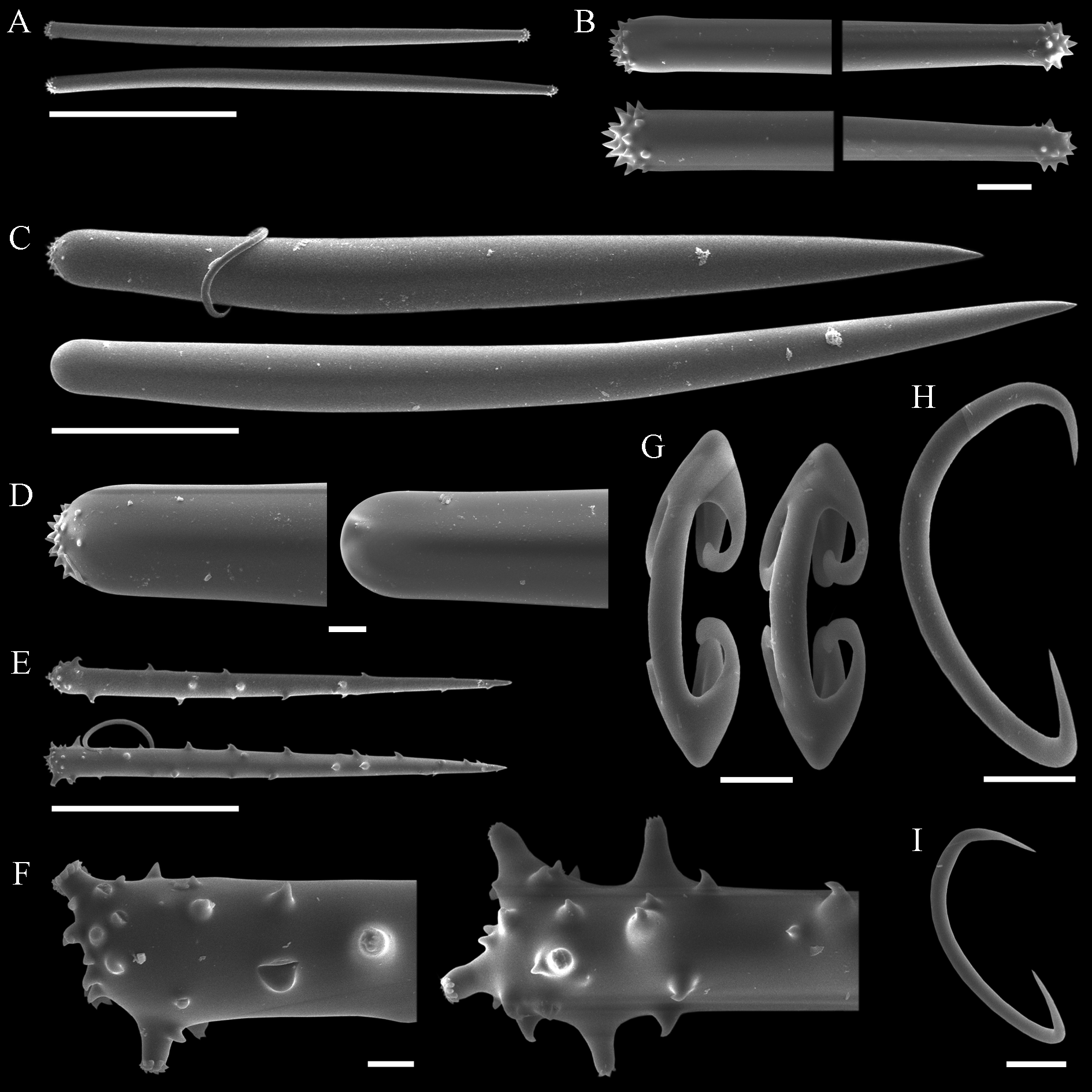
( Tab. 2 View TABLE 2 ; Figs 10–13 View FIGURE 10. A – C View FIGURE 11 View FIGURE 12 View FIGURE 13 )
Holotype. IZUA–POR 169, Isla Diego Ramírez, southern Chile (56ºS, off Cape Horn), ca. 2000 m depth, September 2002, bycatch from longline fisheries, Leg. A. Bravo. Fragment from holotype deposited under MNRJ 10884.
Comparative material. Lissodendoryx ( Ectyodoryx) anacantha (Hentschel, 1914) (ZMH S 2327, fragment from the holotype).
Diagnosis. Seemingly erect Lissodendoryx ( Ectyodoryx) with irregular surface, terminally microspined tylotes (223–252/7.2–9.6), acanthostyles (I. 435–602/24–31, II. 320–415/19–21, III. 220–242/14–17), arcuate isochelae with lateral alae bent towards the shaft (22–24), and sigmas ( I.45 –60, II. 18.6–25).
Description. The holotype is incomplete and therefore imprecise to describe its gross morphology. It is seemingly erect, possibly stalked ( Fig. 10A View FIGURE 10. A – C ), ca. 2 cm high and 0.4 cm thick. Surface rather hispid, irregular, bearing short projections ( Fig. 10B View FIGURE 10. A – C ) and roundish openings (ca. 0.1 cm across), which are of doubtful oscular in function. A gross reticulation is apparent in the entire sponge under transmitted light in a stereo microscope ( Fig. 10C View FIGURE 10. A – C ). Colour in life not reported, dried holotype beige. Consistency relatively compressible and delicate, texture slightly rough.
Skeleton. Plumoreticulate with a slight degree of axial compression ( Figs 11 View FIGURE 11 A–B). Larger acanthostyles core main paucispicular tracts (approx. five spicules across) that are entirely echinated by another two distinct types of acanthostyles ( Fig. 11 View FIGURE 11 C). Some of the larger acanthostyles fan out from the main tracts, interconnecting them. Aniso-subtylotes are scattered at the surface, disposed at various angles to it ( Fig. 11 View FIGURE 11 D). Isochelae and sigmas are scattered in the ectosome and choanosome. There is no spongin enveloping the fibres and subectosomal lacunae are absent. Choanosomal cavities (ca. 50 µm in diameter) are uncommon, scattered and roundish.
Spicules. Megascleres ( Tabs 2 View TABLE 2 –4): Aniso-subtylotes to aniso-strongyles ( Figs 12 View FIGURE 12 A–B), straight, microspined ends , 223– 233.5 (10.2) –252/7.2– 8.4 (1.3) –9.6. Acanthostyles I ( Figs 12 View FIGURE 12 C–D), straight to slightly curved, stout and somewhat fusiform; roundish base, frequently narrower than the central portion of the shaft; gradually thinning point; surface with few straight spines (up to 2.5 µm), mainly over the basal third of the spicule, 435– 536 (52.8) – 602/24– 26.6 (2.4) –31. Acanthostyles II ( Figs 12 View FIGURE 12 E–F), same overall morphology as the preceding category, but smaller, and bearing more spines, although the apical portion is also totally smooth, 320– 391 (29.2) –415/19– 20 (1) –21. Acanthostyles III ( Fig. 12 View FIGURE 12 H) straight to slightly curved; narrow roundish base; gradually thinning point; shaft completely covered with spines (moderately to abundantly), spines frequently straight and usually larger than those of the preceding categories, 220– 232.5 (10) –242/14– 15 (1.1) –17. Microscleres ( Tabs 2 View TABLE 2 –4): Arcuate isochelae ( Fig. 12 View FIGURE 12 I), shaft gently curved, lateral alae elongated and curved towards the shaft as a claw, frontal alae simple, 22– 23.5 (1) –24. Sigmas I ( Fig. 12 View FIGURE 12 J), contorted, smooth, with sharp ends , 45– 55.5 (4.9) –60. Sigmas II ( Fig. 12 View FIGURE 12 K) same morphology as the preceding one, but smaller, 16.8– 20.8 (2.9) –25.
Ecology. Deep-water habitat ( 2000 m depth).
Distribution. Currently only known from the type locality.
Etymology. The species is named after its type locality.
Remarks. Lissodendoryx ( Ectyodoryx) diegoramirezensis sp. nov. is distinguished from Lissodendoryx ( E.)
spp. occurring in the SE Pacific, additional allied biogeographic provinces, as well as L. ( E.) ballena sp. nov., L. ( E.) corrugata sp. nov., and L. ( E.) coloanensis sp. nov., mainly by its possession of three categories of acanthostyles, separable by their dimensions, morphology and placement in the skeletal architecture ( Tab. 2 View TABLE 2 ; Figs 12 View FIGURE 12 C–H).
Lissodendoryx ( Ectyodoryx) diegoramirezensis sp. nov. has arcuate isochelae of uncommon morphology, with lateral alae of both extremities curved towards the shaft ( Fig. 12 View FIGURE 12 I). Precisely this isochelae morphotype can be found in L. ( E.) anacantha (Hentschel, 1914: p.107, Taf. VII, Fig. 12 View FIGURE 12 ), a species amply distributed around Antarctic and the Subantarctic Region, and in another Antarctic species, L. ( Lissodendoryx) styloderma Hentschel, 1914 (Hentschel, 1914: p.101, Taf. VII, Fig. 7 View FIGURE 7 ). This latter species can be readily distinguished from the new species by its lack of sigmas, possession of styles in the ectosome, and acanthostyles of a single category only. However, the similarity of the isochelae of the new species and those seen in L. ( E.) anacantha might indicate a closer affinity between both species, as they further share similar habit and additional spicule characteristics. Nevertheless, both species are considered distinct on the basis of the presence of a third category of acanthostyles in the new species, while its acanthostyles, as a rule, are also more densely spined or with spines more widespread over the shaft. Furthermore, L. ( E.) anacantha has ectosomal megascleres that can be perfect styles ( Fig. 13 View FIGURE 13 C–D), and the smaller category of acanthostyles may bear secondary microspines over the main spines seen at the base of the spicule ( Fig. 13 View FIGURE 13 E–F). These characteristics of the acanthostyles have not been spotted in the holotype of L. ( E.) diegoramirezensis sp. nov. ( Fig. 11 View FIGURE 11 C–H). The re-examination of the type material of L. ( E.) anacantha ( Fig. 13 View FIGURE 13 ) confirmed the spicule set originally reported by Hentschel (1914), and subsequently recognized by Koltun (1964). Nevertheless, new measurements obtained from the holotype ( Tab. 2 View TABLE 2 ) revealed that acanthostyles can be much thicker than reported originally (Hentschel, 1914), matching more closely the measurements reported by Koltun (1964).
Given the data at hand, L. ( E.) diegoramirezensis sp. nov. is considered well distinguished from allied forms, but it is suggested that study of additional samples of L. ( E.) anacantha , such as those registered (but not described) by Burton (1932) from South Georgia, is important to verify if these two taxa are distinct, as hypothesised here.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
|
SubGenus |
Lissodendoryx |