Eudrilus eugeniae ( Kinberg, 1867 )
|
publication ID |
https://doi.org/ 10.5733/afin.056.0302 |
|
publication LSID |
lsid:zoobank.org:pub:CBAD704B-64F6-421B-BC71-74DF4620DB4E |
|
DOI |
https://doi.org/10.5281/zenodo.7670512 |
|
persistent identifier |
https://treatment.plazi.org/id/03F287D3-077A-B10F-FE07-A407F26C701C |
|
treatment provided by |
Felipe |
|
scientific name |
Eudrilus eugeniae ( Kinberg, 1867 ) |
| status |
|
Eudrilus eugeniae ( Kinberg, 1867) View in CoL View at ENA
Figs 1 View Fig –4
Lumbricus eugeniae Kinberg, 1867: 98 . [Type locality: Humid mounts and valley of St Helena (15°56'S 05°43'W). Types in Natural History Museum, London BMNH 1904.10 .5.550 with Swedish Museum label: “ Lumbricus Eugeniae Kinberg St Helena Swed. State Museum.” The specimen was in moderate condition when briefly inspected in June, 2013 (Blakemore 2014: 122)].
Eudrilus decipiens Perrier, 1871: 1176 View in CoL ; 1872: 78, figs 26–30; Horst 1887: 247 (syn.: lacazii View in CoL , peregrinus View in CoL , boyeri View in CoL ). [From Antilles. Types in Paris].
Eudrilus lacazii Perrier, 1872: 75 View in CoL . [ From Martinique (collected 1826). Types in Paris].
Eudrilus peregrinus Perrier, 1872: 77 View in CoL , fig. 76 (of ova). [ From Rio de Janeiro (collected 1833). Types in Paris].
Eudrilus boyeri Beddard, 1886: 302 View in CoL . [ From New Caledonia. Types BMNH 1904 :10:5:612].
Eudrilus sylvicola Beddard, 1887: 372 View in CoL . [ From British Guyana. Types BMNH 1904 :10:20:408].
Eudrilus jullieni Horst, 1890: 225 View in CoL . [ From Liberia. Types in Leiden?].
Eudrilus roseus Michaelsen, 1892: 224 View in CoL , fig. 10. [ From Caracas , VeneZuela. Types Humboldt Museum, Berlin 2162. Michaelsen notes “? Eudrilus perigrinus E. Perr. ” (sic)].
Eudrilus erudiens Ude, 1893: 71 View in CoL . [ From Bermuda. Types?].
Eudrilus eugeniae: Beddard 1895: 604 View in CoL , fig. 30 (syn.: lacazii View in CoL , peregrinus View in CoL , decipiens View in CoL , boyeri View in CoL , sylvicola View in CoL , jullieni View in CoL , roseus View in CoL ); Eisen 1900: 135, figs 27–50, 95–97; Michaelsen 1900: 402 (syn.: decipiens View in CoL , lacazii View in CoL + peregrinus Perrier, 1872 View in CoL ; boyeri View in CoL , sylvicola View in CoL , jullieni View in CoL , roseus View in CoL , erudiens View in CoL ); Stephenson 1923: 486; 1930: 873; Gates 1942: 137; 1972: 51; 1982: 72; Sims & Gerard 1999: 146, fig. 52; Sims 1987: 386; Csuzdi & Pavlicek 2009: 13 (excluding the peregrinus View in CoL synonym by oversight?); Blakemore 1994; 2002; 2012 b; 2013; 2014: 122.
Etymology: Named after Johan Gustaf Hjalmar Kinberg’s Swedish survey ship, the ‘Eugenie’.
Description:
External morphology:
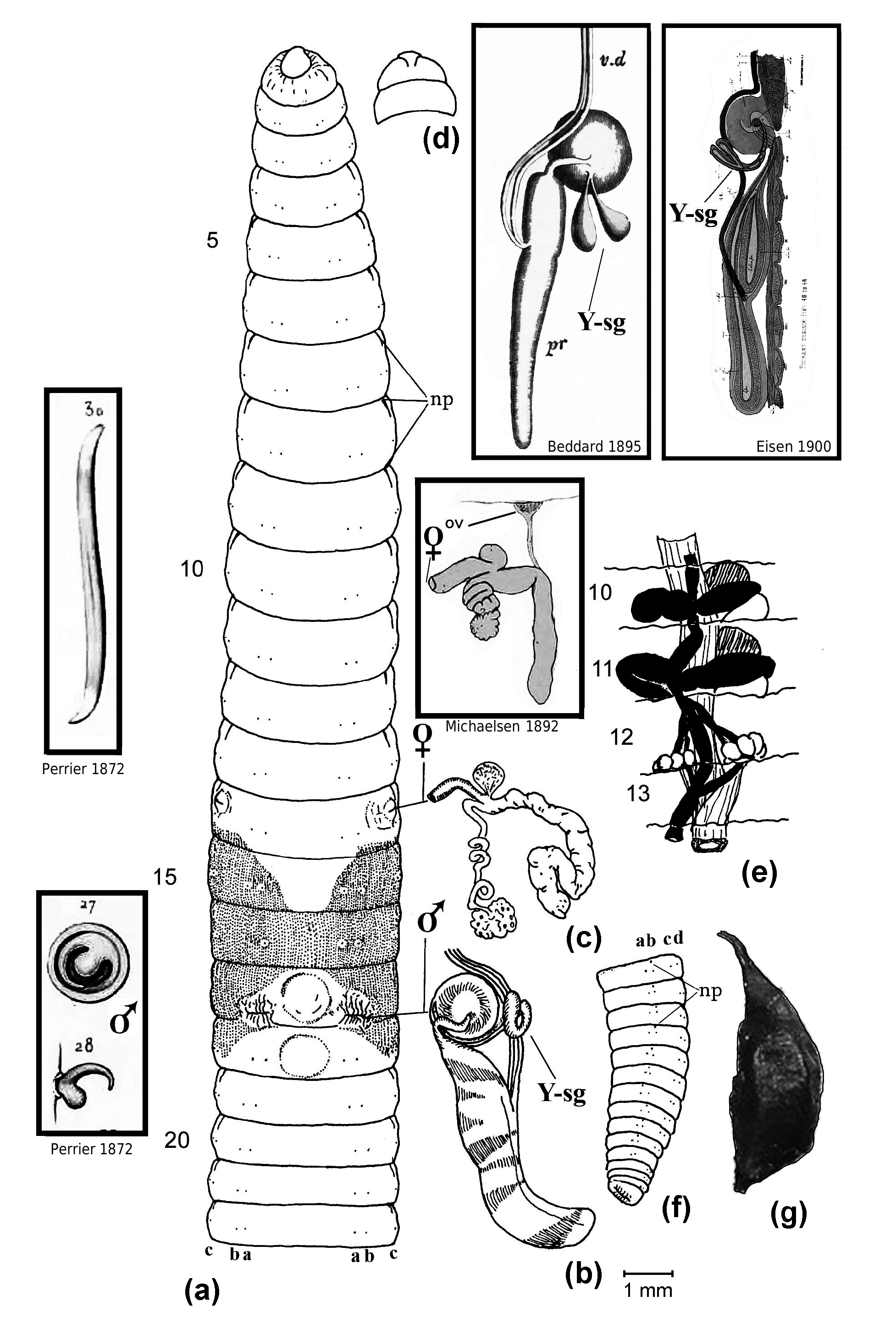
Body length: Complete matures, 90–185 mm (pers. obs. and Gates 1972) or up to 250–400 mm under optimal culture conditions (Viljoen & Reinecke 1994; Parthasarathi 2007); posterior tapering, becoming thinly flattened in terminal ‘Zone of growth’ (Gates 1982). Width: Approximately 4–8 mm.
Mass: Mean per adult ca. 1.0 g (pers. obs.) or optimal maximum 5.0–6.0 g.
Segments: 161–211 (pers. obs. and Gates 1972) or 250–300 (Viljoen & Reinecke 1994, suggesting that larger worms add segments); constriction of 40–46 seen in several Qld specimens may be artefactual.
Colour: Red-brown dorsum fading posteriorly; anterior with bright blue/green iridescent sheen from cuticle diffraction, ventrum beige, clitellum darker (sometimes lighter) than surroundings.
Prostomium: Small, open epilobous.
Dorsal pores: None.
Setae: Eight per segment from 2, closely paired; setae a–b on 17 absent (dehisced); ratio of aa:ab:bc:cd:dd:U on 7 =6:1:5:1:10:0.5. Penial/genital setae absent.
Nephropores: Just behind anterior furrow of each segment (longitudinal slits) from 3/ 4 in c lines or slightly more median (sometimes in d lines).
Clitellum: 13, 14, 15–18, usually 13, 14–18 and interrupted ventrally.
Male pores: In 17 on tips of longitudinally grooved, tapering, eversible penes in large ventral chambers, retracted as lateral slits with wrinkled lips just anterior to 17/ 18 in line with b setae.
Female pores: Combined with modified ‘spermathecal pores’ (see Fig. 2 View Fig ) lateral, presetal in 14 as raised intrasegmental openings just anterior to c setae. Gates (1972: 51) calls these “vaginal apertures”.
Genital markings: Central raised pad centred in 17 between male pores, faintly repeated in 18; sometimes undeveloped or as elliptical, opaque area in 16–18 (Gates 1982).
Internal anatomy:
Septa: From 4/5; (6/)7/8/9 and 14/15 thickened.
Dorsal blood vessel: Single, truncated before anterior hearts in 7; according to Gates (1972: 51) connects to paired supra-oesophageals in 7–14 and paired extra-oesophageals median to the hearts.
Hearts: In 7 lateral, in 8–11 latero-oesphageal, all distended with blood in some Qld specimens (cf. Gates (1972) who said the anterior hearts were undistended).
Gizzard: Weakly muscular in 5 immediately behind pharyngeal mass.
Calciferous glands: Ventral spheroidal sacs in 10 and 11 (concealed by seminal vesicles): large and pink due to blood supply with many internal lamellae; also in 12 (concealed by seminal vesicles) a pair of yellow, lobular ‘calciferous’ glands which are medially placed lateral to the oesophagus and ducted posteriorly into it in 13. This latter pair supplied by quite large blood vessels (from supra-oesophageal vessels). Michaelsen calls the median oesophageal sacs “chylustaschen” but Stephenson (1930) only called the paired glands in 12 “calciferous”. Eisen (1900: 138) found neither crystals nor lime granules in the paired “diverticles” in 12, whereas Gates (1972: 51), after claiming calcareous granules in both median and paired glands, classed them all as calciferous. Intestine: Origin in 14 or close to 14/15. Caeca and typhlosole absent. Small, supraintestinal glands present in eight to forty-two segments in some of 62–132 (Gates 1972: 52; 1982: table 8) may assist digestion and/or be implicated in the immune competency of the worms.
Nephridia: Paired , large coiled holonephridia in each segment from 4, not obviously vesiculate.
Male organs: Holandric with two large, unpaired (or attached?) sacs seen ventrally in 10 and 11, each contain a testis anteriorly and funnels posteriorly, i.e. two pairs of testes in 10 and 11; paired seminal vesicles occupy 11 and 12 and are filled with coagulum. The testes funnels are small and free from iridescent spermatozoa which aggregate in the ducts and thus are easily missed. The male apparatus is complicated and descriptions differ somewhat; the copulatory chamber contains a pointed and curved penis plus a large round papilla or porophore of what Eisen (1900: 140, figs 44, 46) and Gates (1972) describe as a “Y-shaped gland” that opens into a groove going nearly to the tip of the penis. Eisen found the product of this Y-shaped gland to be a secretion similar to that of the silk gland of a caterpillar (possibly analogous to penial setae as found, for example, in Nsukkadrilus mbae Segun, 1977 , to remove sperm of previous concopulant?). The Y-shaped gland is lacking in Eudrilus pallidus Michaelsen, 1891 and the copulatory chambers are absent from E. simplex Michaelsen, 1913 , serving to anatomically separate them from E. eugeniae according to Beddard (1895) and Gates (1972: 51).
Female organs: These are compleX and difficult to characterise correctly. Large eggfilled ovisacs attach to each spermathecal atrium (although Gates (1942: 142) mistakenly calls this the ‘diverticulum’) or duct by long, coiled oviduct tubes in 14, sited opposite a saccular gland. Eisen’s (1900: 139) description differed from Beddard’s (1895) but both (mistakenly?) agreed that ovaries in 13 are combined with ovisacs; and, whereas Eisen thought there were two pairs of ovaries in segment 13, Gates (1972: 52) had the second, functional pair in 14. However, Michaelsen (1892: 225, fig. 10) clearly showed small ovaries paired behind septum 12/13 connecting with the saccular part of the spermatheca (what Sims (1987) calls the “receptaculum seminis”) and that the ovisac or “receptaculum ovorum” is terminal to a long second oviduct. Easily missed, this smaller oviduct connection to the spermatheca was figured by Eisen (1900: figs 49–50) and reported by Gates (1942: 142) although Sims (1964: 303, fig. 6) says the small oviduct usually connects with the larger oviduct leading to the ovisac where the eggs mature (as described by Eisen 1900: 139). Histological sections of Vijaya et al. (2012) showed a dense mass of sperm in the oviduct they took to confirm internal fertilisation, supporting its classification by Sims (1964) as a “fertiliZation chamber” rather than a spermatheca. Gates (1972: 51) calls it a vagina whereas Segun (1977: 261, fig. 2) uses the terms “ovo-spermathecal duct” and “ovarian vesicle”.
Spermathecae: As just noted under ‘Female organs’, there is an atrium with muscular sheen in 14 that eXtends into a long flaccid, convoluted gland, filled with coagulum and enclosed in a sheath; at their junction a long oviduct attaches leading to the ovisac which is opposed by a small saccular outgrowth. The whole or just part of the structure may be referred to as a ‘fertilisation chamber’ as it functions for internal fertilisation of eggs with sperm, presumably before transfer of the embryos to the cocoon.
Prostates: Large pair of digitiform euprostates, with white muscular sheen from 18 extending to 23; acutely muscular enlargements of loop of paired sperm ducts which attach to apex of copulatory chamber mound centrally. As noted, a smaller blind duct — the Y-shaped gland — attaches to the base of the mound mesially, although Beddard (1895: fig. 30) shows a pair of such glands.
Other internal features: Small saccular ‘brown bodies’ formed from coelomocytes were observed loose in coelomic cavities from 7 posteriorly; these may enclose shed setal follicles (as also noted by Gates 1972: 52). Beddard (1891: figs 2–3) reported and figured sensory glands in the mid-body that he called “pacinian bodies” which Eisen (1900: 143, fig. 95–97) decided were partly sensory structures to detect sound as “primitive auditory organs” equivalent to otosomes found in Pontoscolex ; the function in both cases is unknown.
The gut contains soil and/or organic matter (depending on habitat) — this species appears to be an adaptive feeder and will survive in unaltered soil (as noted) but also flourishes on organic material.
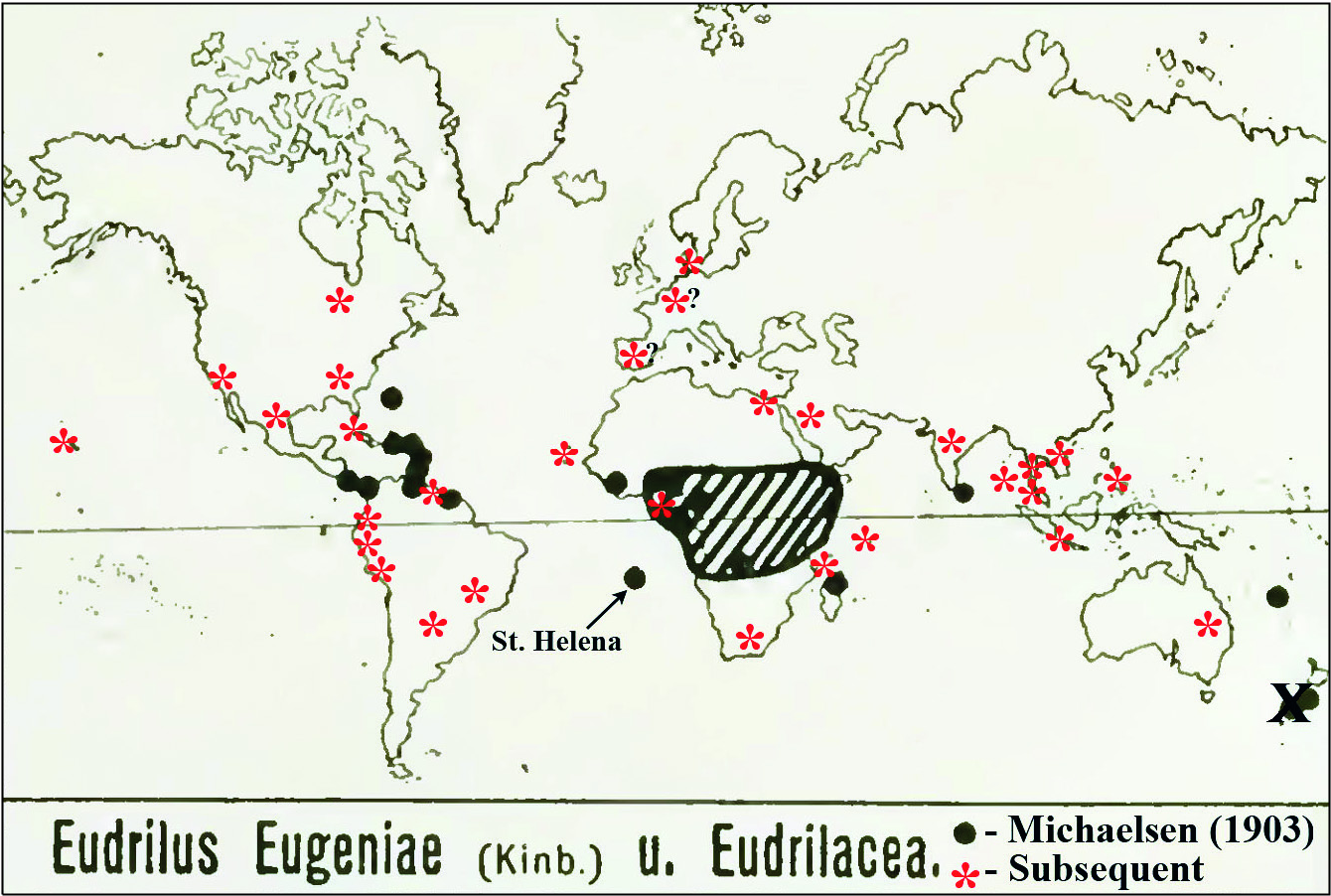
Cocoons: Dark coloured with adhesions, tapered lemon-shape with one side usually being flatter, mean siZe approX. 6× 3 mm (from Reineke & Viljoen 1988, who also provide incubation and hatching data); may contain from one to eight hatchlings (Gates 1982). Distribution ( Fig. 3 View Fig ): After Michaelsen (1903: 122); Gates (1942: 98, 1972: 52, 1982: 72): West African origin from Upper Guinea plain or coastal forest including Sierra Leone, Liberia, Ivory Coast, Ghana, Togoland ( Benin), Nigeria, Cameroon, Gabon and the Congo; transported and peregrine to many tropical countries such as Madagascar and the Comoros Islands (e.g. Anjouan), Seychelles ( Gerlach 2011), Sri Lanka and India (Michaelsen 1903; Stephenson 1923: 486; Dhiman & Battish 2005), and New Caledonia; the Americas: [e.g. Gates (1982: 74) said it owes its North American distribution since the 1950s solely to the fishing bait market having been shipped into every one of the lower 48 United States, such as Florida, Alabama, Georgia, Texas, and even to Hawaii, as well as several Canadian provinces]; Central and South America, e.g. MeXico ( Rodriguez-Aragones 1999), Suriname ( Horst 1887), Panama [from 1896 — Eisen (1900: 135) said: “Judging from the number of specimens in the collection, this species must be the most common of the large terrestrial earthworms in Panama ”], BeliZe (also as an introduction from the then ‘British Honduras’ noted by Gates 1982), Venezuela (e.g. roseus ), Guyana, Colombia ( Feijoo et al. 2004), Paraguay ( Schuldt 2009), Brazil; the Caribbean: e.g. Haiti, Trinidad, Martinique, St Thomas, St CroiX, Puerto Rico, Virgin Islands (Michaelsen 1903, 1910; Gates 1942, 1972), Cuba (Gates 1972; Alvarez & Rodriguez-Aragones 2010), Bahamas, Antilles ( Gates 1942: 99) and Guadeloupe ( Csuzdi & Pavlicek 2009 — who found it in a natural setting indicating it may have become feral there as it is on St Helena); also the Atlantic: Bermuda (as E. erudiens ), St Helena (type-locality by introduction), Cape Verde (from where it was introduced to New York (Gates 1982), Fernando Po [Bioko] and São Tomé Islands (Michaelsen 1903, 1910). Elsewhere in America, Gates (1982: 72–74) explained in some detail how the first report from the US mainland was in 1950 from “Lake Geneva, Florida ” from a “can of worms (bait) inadvertently left behind” and cultured by the camp owner (a Mr T. Baker), eventually shipped to all of the USA and Canada where it has been cultured both indoors and outdoors.
The first Australasian taXonomic confirmation was from near Brisbane, Queensland in 1991 ( Blakemore 1994, 1999) with stock (surface sterilised cocoons) originally obtained from Canada (Mr G. Bosanquet pers. comm. 1991).
In Europe it was introduced to Hamburg with plants from the West Indies (Michaelsen 1903: 12) and to Kew Gardens in Wardian cases from British Guiana (Beddard 1906). It is rarely reported from northern European glasshouses by Sims and Gerard (1985), albeit rarely, e.g. from Denmark (Blakemore 2007) and eastern Europe, Hungary ( Csuzdi et al. 2007); also maintained in laboratory cultures, e.g. Vigo, Spain ( Dominguez et al. 2001).
Plisko (2010) notes that it was deliberately introduced to South Africa ( RSA) by Reinecke and Viljoen (1988) from Germany in stock originating in West Africa and that this species is now widely used in RSA farms and is “adapting well to habitats in this country” suggesting its naturalisation there.
Eudrilus eugeniae View in CoL is stated to be newly introduced to Egypt ( Medany & Yahia 2011: 20), but what this paper actually says is: “Four types of earthworms were brought to Egypt from Australia: Lumbriscus Rubellus (Red Worm) , Eisenia Fetida (Tiger Worm) View in CoL , Perionyx Excavatus (Indian Blue) , and Eudrilus Eugeniae View in CoL (African Night Crawler)”. However, Lumbricus rubellus Hoffmeister, 1843 View in CoL has never been proven a vermicomposting worm (Blakemore 1999, 2002), thus it is likely only three species or fewer were involved. Eudrilus View in CoL is newly demonstrated in vermicompost and aquaponics filters in Jeddah, Saudi Arabia ( Alamoodi 2014).
At least one worm farmer in Valparaiso, Chile and a technician (Mr Reinaldo Plasencia) in Nicaragua claim to rear Eudrilus View in CoL (“la lombriz africana”) sometimes misspelled “ Fudrillus spp” ( Lumbricultura 2014; Monographias 2014), which would both be new national reports. Mr Enzo Bollo Tapia (pers. comm. 2014) communicated that it can be cultivated in Ecuador, Colombia and Peru but that Chile is unsuitable for its survival due to climate, although he did experiment there.
Introduced to the Philippines for vermicomposting in the 1980s, E. eugeniae is now distributed in worm-beds on farms over the whole country. A report of its spreading to some mountainous inland areas via agro-forest strips of Negros Occidental by Flores (2007) is unsubstantiated as there is no proof that E. eugeniae itself was found. The report just says “ Eudrilus ” based on a novice’s key to families. It is also newly reported from Thailand, from an unpublished DNA barcode submission to GenBank in 2010/2011 (see Appendix) and recent reports from there (e.g. Malliga 2010; Loongyaii et al. 2011). Eudrilus is used for soy bean residues and rice husks vermicomposting in Malaysia (e.g. Lim et al. 2011; Shak et al. 2014) with the worms apparently imported as cocoons from India. It is also reported from Indonesia where vermiculture operations in Solo, Central Java are advertised (e.g. Indonetwork 2014; Cacinglumbricus 2014). This has now been confirmed by the Animal Husbandry Faculty at Bogor Agricultural University, West Java (Andy Darmawan pers. comm. via email Nov. 2014). Recent reports from Vietnam are from the provinces of Lang Son and Cao Bang by the Research Institute for Aquaculture [ The Anh et al. (2011); AFSPAN (2012) but mispelt “ Eudrilus euganaie ”].
New Zealand records by Beddard (1895: 149), repeated by Michaelsen (1900, 1903), Hutton (1904: 355), Gates (1972), and Sims and Easton (1985) were stated by Thompson (1922: 359), Benham (1950) and Lee (1959: 365) to be an error introduced when Beddard (1891, 1895: 149) somehow mistook for Eudrilus eugeniae Smith’s 1886 report of Endrilus [sic lapsus for Eudrilus ] levis [= Octochaetus ? levis (Hutton, 1877)] from Taranaki. Recent personal surveys of vermicomposting operations in New Zealand also failed to locate this species there (e.g. Blakemore 2012 a).
The claim from the French islands off the coast of Newfoundland ( St Pierre and Miquelon) of E. lacazii by Perrier (1872) was disputed by Gates (1982: 72), although this is possibly Gates’s mistake as its type locality is Martinique in the Antilles, where there is also a town named St Pierre, rather than the one near Miquelon. No records of cultivation are confirmed from Germany (the supposed source some worms in the Philippines and South Africa), from a few southeast Asian countries neighboring Vietnam, or yet from China / Taiwan.
Locality: Specimens were collected from worm farms in Brisbane (1991) and samples sent to the author from Mackay, Qld (1992), and Menai, NSW (1996) [now in CSIRO/ ANIC, Canberra with registration nos. RB.95.9.4/11.2 (Blakemore 1995)]; also confirmed from lowland Philippines (specimens in Fishery collection of UPV, Miagao) but only close to worm beds; neither was it located ferally in surveys on Negros Island (pers. obs. 2009–2014, cf. Flores 2007).
Habitat: Originating in shaded savannahs of West Africa , it now thrives in worm beds on worm farms; it is reported in natural high moisture/organic sites such as waterfalls or riverbanks on Guadeloupe and also in gardens and some vegetable or fruit fields in South America ( Brown & Fragoso 2007: 372). It is newly found in vermifilters of aquaponics tanks at Sulu Gardens in Miagao , Philippines (pers. obs. Feb. 2014).
Behaviour: Hatchlings are reported to sometimes return to the cocoon when alarmed. Active with a rapid escape response when disturbed, but if captured the adult worms become placid and can be readily handled. The species will wander at night, leaving plant pots and escaping unsealed containers when there is no light source. For rapid field identification, slight pressure between the fingers will cause eversion of white penes that are shaped similar to a scorpion’s stinger (see Fig. 2 View Fig ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Eudrilus eugeniae ( Kinberg, 1867 )
| Blakemore, Robert J. 2015 |
Eudrilus erudiens
| UDE, H. 1893: 71 |
Eudrilus roseus
| MICHAELSEN, W. 1892: 224 |
Eudrilus boyeri
| BEDDARD, F. E. 1886: 302 |
Eudrilus decipiens
| HORST, R. 1887: 247 |
| Perrier 1871: 1176 |
Lumbricus eugeniae
| KINBERG, J. G. H. 1867: 98 |