Xenobolus carnifex ( Fabricius, 1775 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4780.1.8 |
|
publication LSID |
lsid:zoobank.org:pub:11054D3F-A1A3-4B78-9F9A-248AAE77F6D7 |
|
DOI |
https://doi.org/10.5281/zenodo.3853023 |
|
persistent identifier |
https://treatment.plazi.org/id/03F48795-FFD2-FFA7-37A6-FC6AFC60A089 |
|
treatment provided by |
Plazi |
|
scientific name |
Xenobolus carnifex ( Fabricius, 1775 ) |
| status |
|
Xenobolus carnifex ( Fabricius, 1775) View in CoL
( Figs 1–9 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 )
Iulus View in CoL (recte: Julus View in CoL ) carnifex Fabricius, 1775: 428 View in CoL (brief description of the species).
Iulus (sic!) carnifex -- Fabricius, 1781: 530 (brief description of the species).
Julus Carnifex View in CoL (sic!)-- Linnaeus, 1788: 3019 (brief description of the species).
Julus carnifex -- Fabricius, 1781: 530 (brief description of the species); 1793: 395 (brief description of the species). Olivier, 1792: 416 (brief description of the species). Latreille, 1802: 76 (brief description of the species). Brandt, 1833: 205 (species name mentioned); 1841a: 127 (species name mentioned); 1841c: 121 (species name mentioned). Humbert, 1865: 57 (brief description of the species).
Iulus (sic!) carnifex -- Gervais, 1837: 13 (species name mentioned); 1847: 163 (brief description of the species). Lucas, 1842: 532 (brief description of the species).
Julus (Spirobolus) carnifex -- Brandt, 1841b: 368 (species name mentioned); 1841c: 121 (species name mentioned).
Spirobolus ruficollis Newport, 1844a: 269 View in CoL (brief description of the species), synonymised by Pocock (1892).
Spirobolus ruficollis View in CoL -- Newport, 1844b: 13 (species name mentioned). Preudhomme de Borre, 1884: 76 (species name mentioned). Attems, 1914: 355 (species name mentioned).
Iulus ruficollis -- Gervais, 1847: 175 (brief description of the species).
Spirobolus Carnifex View in CoL (sic!)-- Koch, 1847: 102 (species name mentioned); 1863: 62, plate XXVII, fig. 53a–b (brief description and illustrations of the species).
Spirobolus carnifex -- Karsch, 1881: 79 (species name mentioned). Preudhomme de Borre, 1884: 73 (species name mentioned). Tömösváry, 1885: 69 (species name mentioned). Daday, 1889: 129 (species name mentioned). Pocock, 1892: 166, plate II, fig. 9 (description and illustration of the species; synonymy of S. ruficollis with X. carnifex ).
Trigoniulus carnifex -- Silvestri, 1896: 28 (species name mentioned).
Diaphoropus carnifex View in CoL -- Silvestri, 1897: 651 (synonymised by Hoffman (1962)).
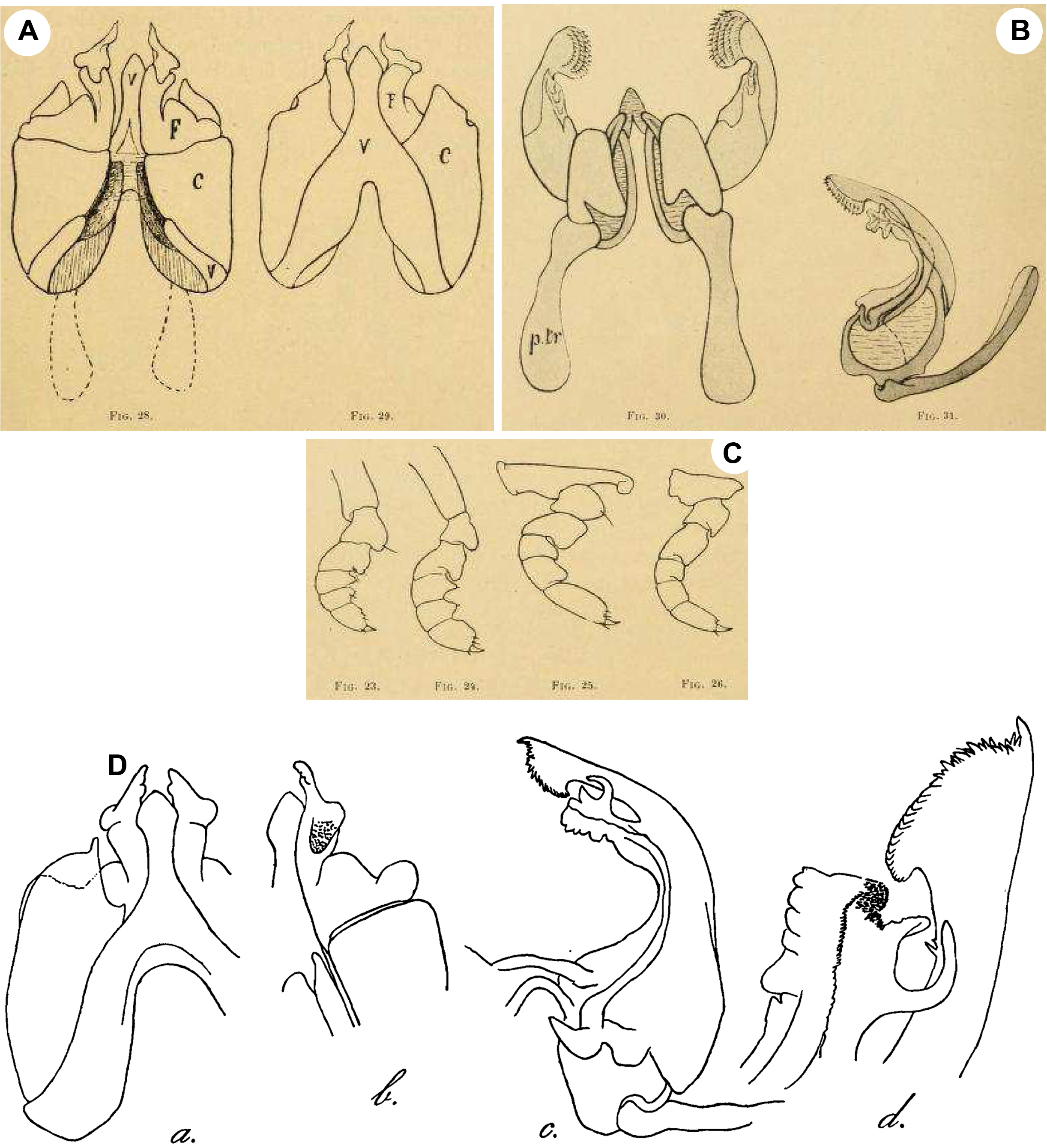
Xenobolus carnifex View in CoL -- Carl, 1919: 394, figs 23–31 (description and illustrations of male). Attems, 1936: 304 (species name mentioned). Ganapati & Narasimhamurti, 1960: 581 (study on gut protozoa). Hoffman, 1962: 780 (species name mentioned; synonymy of E. phoenix View in CoL with X. carnifex View in CoL ). Mukherjee, 1962: 25 (segment variation study). Majumdar, 1967: 109 (study on gut protozoa). Rajulu, 1970: 143 (species name mentioned). Bano, 1983: 170 (species name mentioned). Achar, 1987: 149 (cytogenetic study). Alagesan & Vanithapriya, 1992: 299 (study on the biodigestion of millipede waste for biogas production). Valli, 1994: 322 (toxicity study). Jeekel, 2001: 54 (species name mentioned).Alagesan et al., 2003: 111 (study on gut bacteria). Alagesan & Muthukrishnan, 2003: 313 (study on the effect of temperature on embryology); 2005a: 5 (composition and biomass study); 2005b: 3 (bioenergetics study); 2009: 237 (study of population dynamics). Wesener et al., 2008: 38 (brief description of the species). Wesener & Enghoff, 2009: 110, figs 23D, 24B, 25A–C (illustrations and SEM images of male and female). Karthigeyan & Alagesan, 2011: 62 (millicompost study). Alagesan & Ramanathan, 2013: 1, Fig. 3 View FIGURE 3 (brief description and image of the species); Bhakat, 2014: 185–186, 189–191 (water content analysis). Barber, 2015: 52, figs 1–3 (species name mentioned; images of the species). Chezhiyan & Prabakaran, 2016: 91, plate 2 (species name mentioned; image of the species). De Zoysa et al., 2016: 457 View Cited Treatment (species name mentioned). Golovatch & Wesener 2016: 34 View Cited Treatment (species name mentioned).
Erythroprosopon phoenix Verhoeff, 1936: 505 View in CoL (description of the species), synonymised by Hoffman (1962).
Erythroprosopon phoenix View in CoL -- Moritz & Fischer, 1975: 251 (species name mentioned).
Xenobolus acuticonus Attems, 1936: 303 View in CoL , fig. 87a–d (description and illustrations of the species). New synonymy
Xenobolus acuticonus View in CoL -- Natarajan, 1959: 92 (species name mentioned). Hoffman, 1962: 780 (suggested possible synonymy with X. carnifex View in CoL ). Nagarbhushanam & Gokhale, 1968: 133 (species name mentioned). Rajulu, 1970: 143 (species name mentioned). Sharma & Handa, 1974: 678 (species name mentioned). Demange, 1977: 231 (species name mentioned). Chowdaiah & Kanaka, 1979: 17 (species name mentioned). Janardanan & Ramachandran, 1982: 239 (species name mentioned). Rangaswamy et al., 1983: 8 (species name mentioned). Bano, 1983: 170 (species name mentioned). Achar, 1987: 149 (cytogenetic study). Bai & Indra, 1997: 231 (brief description and natural history of the species). Jeekel, 2001: 54 (species name mentioned). Basil-Rose et al., 2014: 331 (study of hemagglutinability). Golovatch & Wesener 2016: 34 View Cited Treatment (species name mentioned).
Type materials. Type (? ♂ / ♀) of Iulus (recte: Julus ) carnifex from INDIA; D. Koenig leg.; date unknown; voucher number unknown; repository Zoological Museum, University of Copenhagen, not examined as the type material is lost (Enghoff, pers. comm.) [the original illustrations of X. carnifex given in Carl (1919) and the illustrations and SEM images of non-type material of X. carnifex presented by Wesener and Enghoff (2009) are diagnosable and were used for comparative purposes]. Type (♂) of Xenobolus acuticonus from INDIA: Madras; F.H. Gravely leg.; 22 August; voucher number unknown; repository possibly Indian Museum, Kolkata, not examined as the type is currently not in the myriapod collection kept in the National Zoological Collection, Kolkata (Sankaran, pers. obs.).
Topotype material ( X. acuticonus ) examined. INDIA, Tamil Nadu: Chennai, Tambaram, Madras Christian College campus (12 o 55’16.05’’N, 80 o 07’19.42’’E), 41 m alt., 9 December 2018, M.S Pradeep leg., from ground, by hand: 1 subadult ♀ (MILLI-ADSH0010). Chennai, Nungambakkam, Loyola College campus (13 o 03’48.14’’N, 80 o 14’04.36’’E), 15 m alt., 10 December 2018, M.S Pradeep leg., from ground, by hand: 1 ♀ (MILLI-ADSH0011). Chennai, Tambaram (12 o 55’29.75’’N, 80 o 06’00.01’’E), 19 m alt., 21 September 2019, M.S Pradeep leg., from ground, by hand: 6 ♀♀ (MILLI-ADSH0012) GoogleMaps .
Other material ( X. carnifex ) examined. INDIA, Kerala: Palakkad, Thrippalur, Pullodu (10 o 38’16.58’’N, 76 o 33’52.87’’E), 70 m alt., 30 July 2017, M.S. Pradeep leg., from walls, by hand: 7 ♂♂, 5 ♀♀ (MILLI-ADSH0007). Ernakulam,Aluva, Thottakkattukara aqueduct (10 o 07’10.35’’N, 76 o 20’34.62’’E), 11 m alt., 23 July 2017, Jimmy Paul leg., from ground, by hand: 1 ♂, 1 ♀ (MILLI-ADSH0008). Ernakulam, Tripunithura, Hill Palace (9 o 57’09.32’’N, 76 o 21’50.13’’E), 29 m alt., 21 July 2018, Jithin Johnson leg., from bark, by hand: 1 ♀ (MILLI-ADSH0009) GoogleMaps . Andhra Pradesh: Vijayawada , near railway station (16 o 30’54’’N, 80 o 37’22’’E), 30 m alt., 10 October 2019, M.S. Pradeep leg., from walls, by hand: 2 ♀♀ (MILLI-ADSH0013) GoogleMaps .
Diagnosis. As in the generic diagnosis.
Redescription. Measurements: male with 50 body rings, circa 76 mm long, 5.4 mm wide. Female with 50 body rings, circa 76 mm long, 5.8 wide.
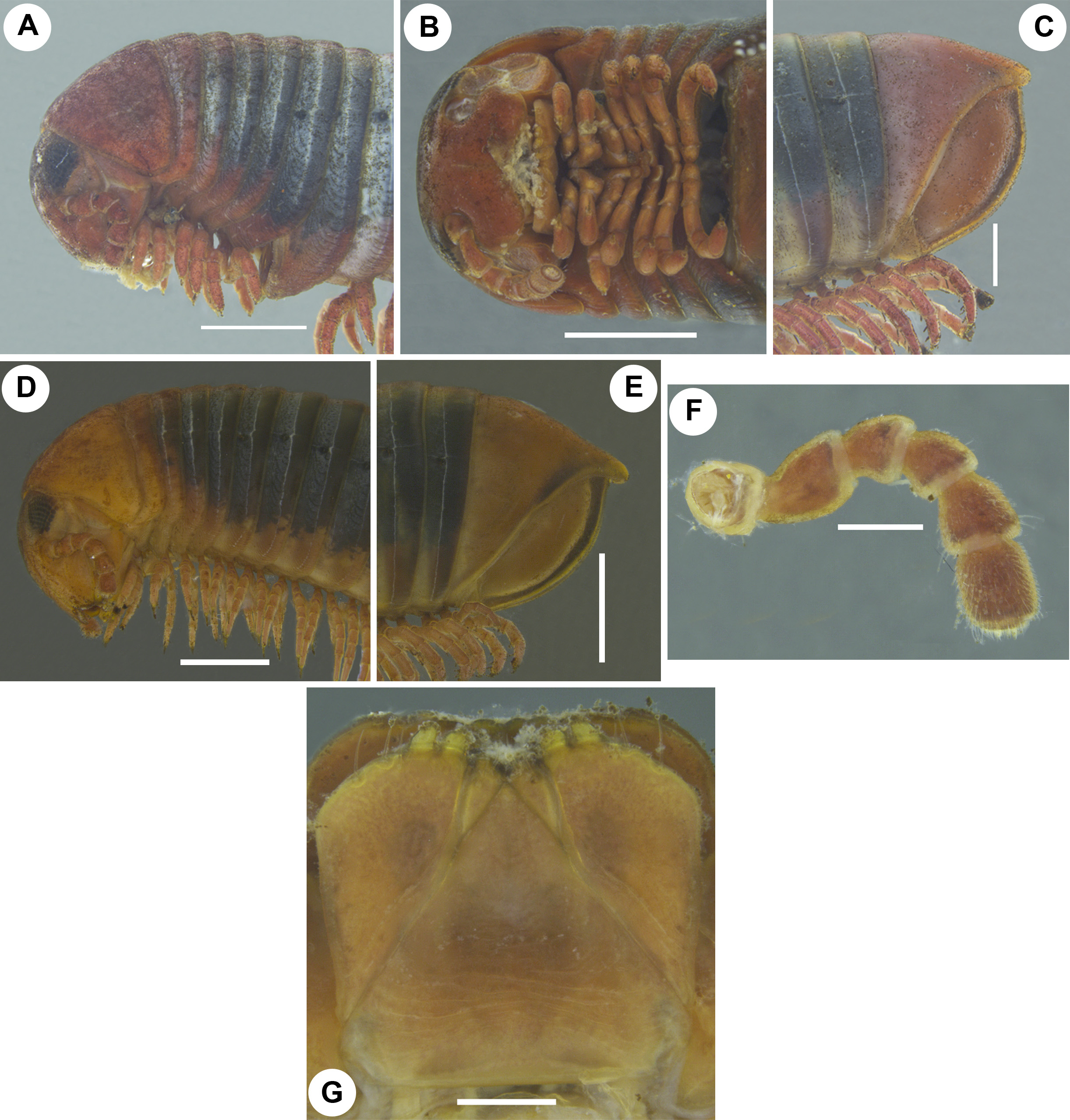
Colour. Aposematic colour pattern consisting blood-red (male)/orange (female) and black ( Fig. 1 View FIGURE 1 A–B). Head, antennae, collum, ring 2, pre-anal ring, anal valves, legs blood-red/orange. Vertex between eyes black. Body rings except ring 2 laterally black, latero-basally and ventrally with a blood-red/orange stripe interrupted at posterior margin of metazonites with irregular creamy and black patches. Metazonites dorsally with a median blood-red/or- ange stripe consisting of an hour glass-shaped pattern ( Fig. 1 View FIGURE 1 A–B). Colouration of preserved material: parts with blood-red/orange colour faded to yellowish red/yellowish orange. Metazonites greyish with irregular creamy-white markings.
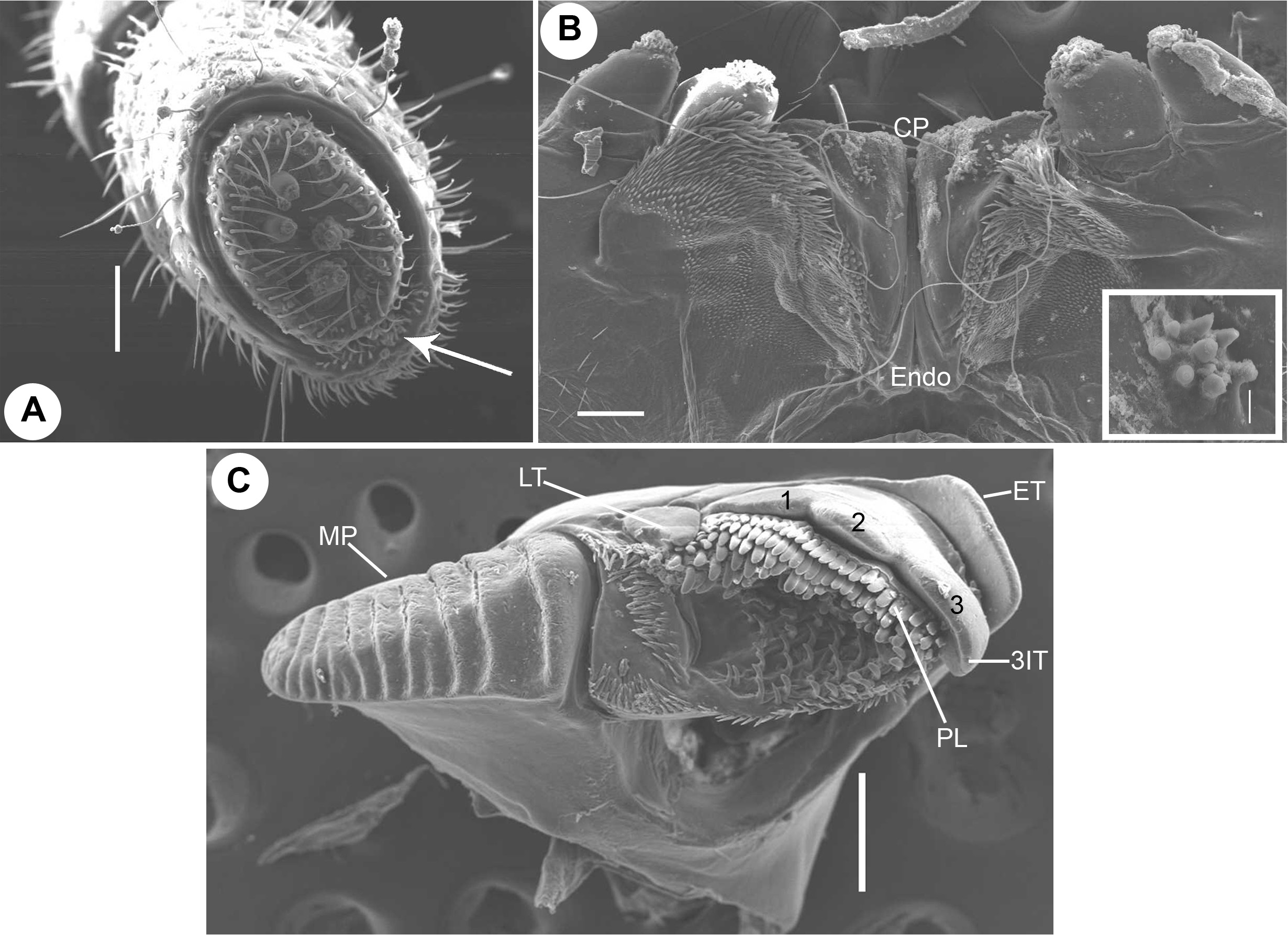
Head. Head micropunctate, vertex prominent. Each eye patch with circa 48–50 ommatidia arranged in 7 or 8 vertical rows. Axial sulcus prominent, discontinued at frons. Labrum with four irregular teeth and a single row of 8 or 9 short marginal setae ( Fig. 2G View FIGURE 2 ). Clypeus with four setiferous foveolae, two on each side. Antennal cavity present, slightly extending below eyes. Antennae short ( Fig. 2F View FIGURE 2 ), protruding back to ring 2. Relative length of antennomeres: 1<2>3>4<5<6. Terminal antennomere with four large sensory cones located together inside a membranous area ( Fig. 4A View FIGURE 4 ). Antennomere 5 apico-laterally with a field of 3 or 4 rows, antennomere 6 with 2 or 3 rows, of sensilla basiconica ( Fig. 4A View FIGURE 4 , arrow).
Gnathochilarium. Usual for spirobolidans. Each lamella lingualis with two setae, located obliquely behind one another. Stipites with a slightly wavy lateral margin, each stipes with three stout apical setae ( Fig. 2G View FIGURE 2 ) and an oblique transverse ridge directed towards mentum ( Fig. 2G View FIGURE 2 ). Basal 2/3 mentum with several transverse ridges ( Fig. 2G View FIGURE 2 ). Palpi with numerous sensilla ( Fig. 2G View FIGURE 2 ). Hypopharyngeal crest with a field of spine-like structures ( Fig. 4B View FIGURE 4 ). Central pads of endochilarium divided by a groove and a ridge into two separate regions ( Fig. 4B View FIGURE 4 ; CP, Endo), distomesally with a group of circa 7 or 8 sensilla arranged in a circle ( Fig. 4B View FIGURE 4 , inset), basally with more than 22 sensilla arranged in oblique vertical rows ( Fig. 4B View FIGURE 4 ).
Mandible. Slim and elongated. External tooth simple, squarish ( Fig. 4C View FIGURE 4 ; ET); inner tooth with three cusps ( Fig. 4C View FIGURE 4 , 1 View FIGURE 1 , 2 View FIGURE 2 , 3; 3 View FIGURE 3 IT), laterally an additional, isolated, simple tooth ( Fig. 4C View FIGURE 4 ; LT). Pectinate lamellae arranged in 5 or 6 rows ( Fig. 4C View FIGURE 4 ; PL). Mesal margin of pectinate area with a single row of small, slender spines continued with circa 4 or 5 rows of small spines baso-mesally ( Fig. 4C View FIGURE 4 ); basal margin with 3 or 4 transverse rows of small spines ( Fig. 4C View FIGURE 4 ). Molar plate long with>10 transverse furrows ( Fig. 4C View FIGURE 4 ; MP).
Collum. Lateral margin rounded, not reaching the tips of ring 2, surface weakly punctate ( Fig. 2A, D View FIGURE 2 ).
Body rings. Divided by sutures in three transverse zones, pro-, meso- and metazona. Meso- and metazonae dorsally micropunctate, ventrally with numerous weak longitudinal and oblique striations. Ozopore located on mesozona, starting with ring 6, located close to, but not touching suture between meso- and metazona. Pre-anal ring/epiproct sharp-edged, slightly extending beyond anal valves/paraprocts, more prominent in female ( Fig. 2C, E View FIGURE 2 ). Anal valves with well-developed lips and micropunctuations, but with neither grooves nor setae ( Fig. 2C, E View FIGURE 2 ). Subanal scale/hypoproct inconspicuous, widely triangular.
Legs. Coxae 1 and 2 elongated and fused with sternites. Coxae 1 unfused together, following ones remain adjacent ( Fig. 3A View FIGURE 3 ). Leg-pair 2 longer than 1st ( Fig. 3B View FIGURE 3 ); each podomere with apical/ventral/mesal stout and slender setae. Length of midbody legs circa 4.9 mm in males, circa 4.92 mm in females. Leg-pair 8 onwards in male and all legs in female, podomeres from coxa to tibia subdistally or distally with a single ventral spine. Tarsus with one stout dorso-apical and 3 or 4 ventral/ventro-mesal stout/slender spines in males, 4 or 5 ventral/ventro-mesal ones in females. Claw large, curved.
Male sexual characters. Coxae 3–7 modified, in particular 3rd and 4th with long, flat, rod-shaped, antero-mesal processes, 5th with a short, flat process, 6th and 7th with narrow, flat prominences ( Fig. 3 View FIGURE 3 C–G). Podomeres from prefemur to tibia of leg-pairs 1–6 and prefemur to postfemur of 7th modified with flat ventral excrescences ( Fig. 3 View FIGURE 3 A–G). Only body ring 7 conspicuously enlarged ( Fig. 2A View FIGURE 2 ). Tips of gonopods visible in ventral view. Legs without tarsal pads.
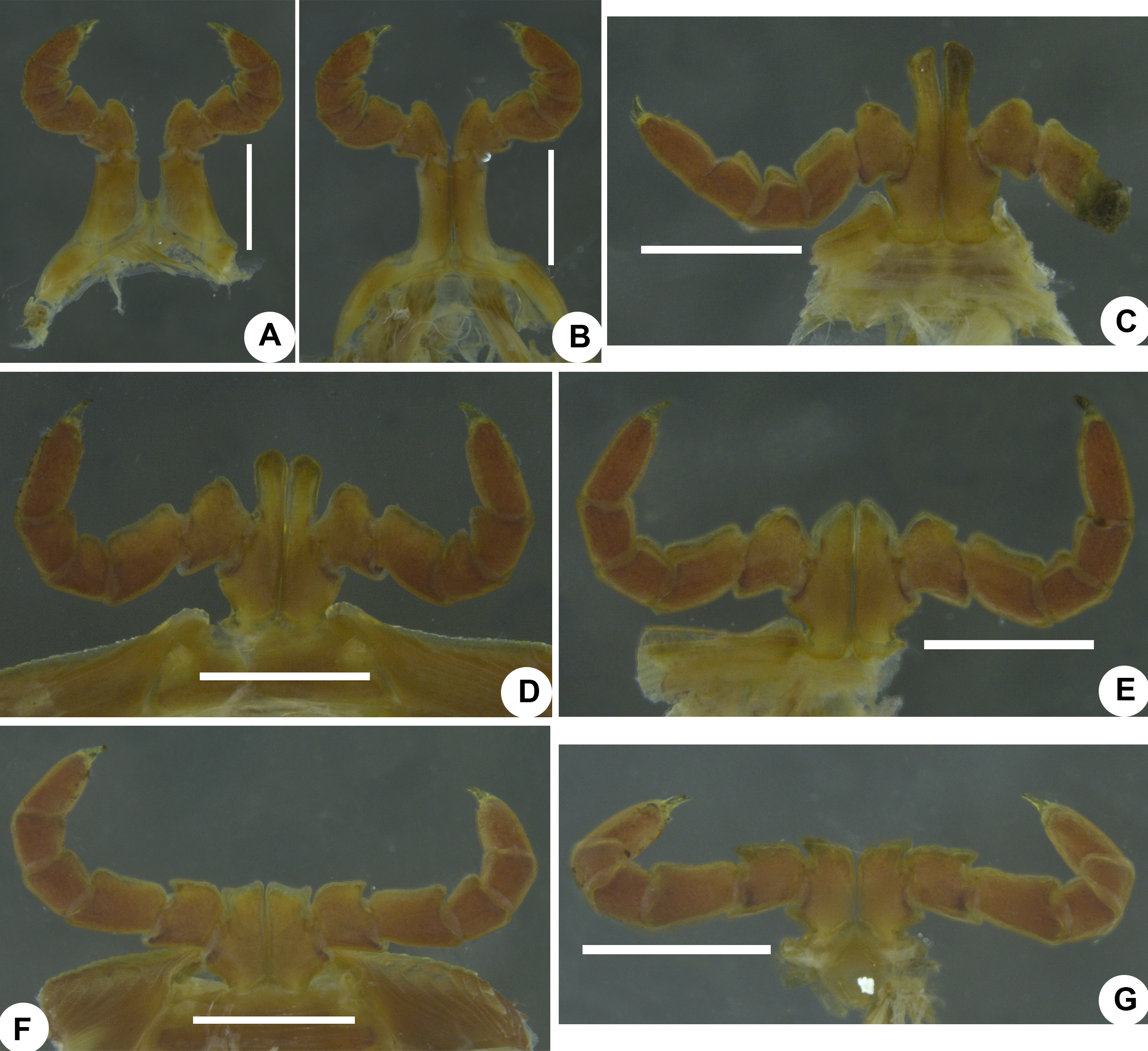
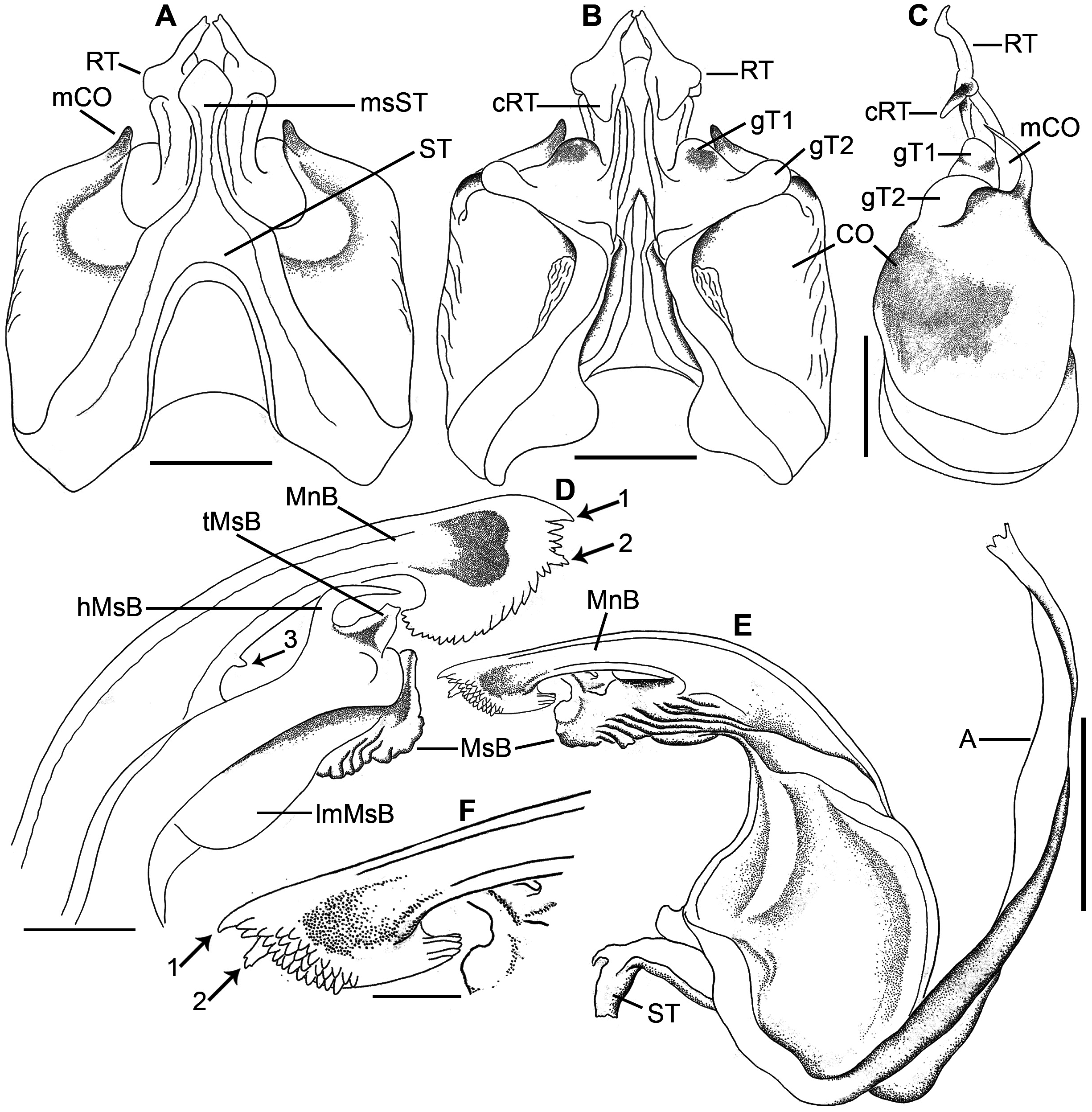
Anterior gonopod. Sternite inverted V-shaped, with a median spatula-shaped process ( Figs 5A View FIGURE 5 , 6A View FIGURE 6 ; ST, msST). Coxite broad, with a thumb-shaped mesal process, flat in lateral view ( Figs 5A, B View FIGURE 5 , 6 View FIGURE 6 A–C; CO, mCO). Telopodite medio-laterally with paired globular processes ( Figs 5B View FIGURE 5 , 6B View FIGURE 6 ; gT1, gT2); retrorse process short, with a flat proximal half, distal half with a downwards directed, broad, conical process and a finger-shaped, laterally oriented, mesal process with apical bifurcations ( Figs 5A, B View FIGURE 5 , 6A, B View FIGURE 6 ; RT, cRT).
Posterior gonopod. Sternite narrow ( Fig. 6E View FIGURE 6 ; ST). Main branch slender; tip broad, semi-circular in outline, fringed with numerous tiny conical processes, with a short, claw-shaped, mesal process, with a short branched process lying adjacent to the claw-shaped process, with a short conical process ( Fig. 6 View FIGURE 6 D–F, arrows 1, 2, 3; MnB). Mesal branch broad, proximo-laterally with a broad, semi-circular, membranous lamella, ridged disto-laterally, mesally with a membranous, trapezoid process and a sharp hook ( Fig. 6D View FIGURE 6 ; MsB, lmMsB, tMsB, hMsB).
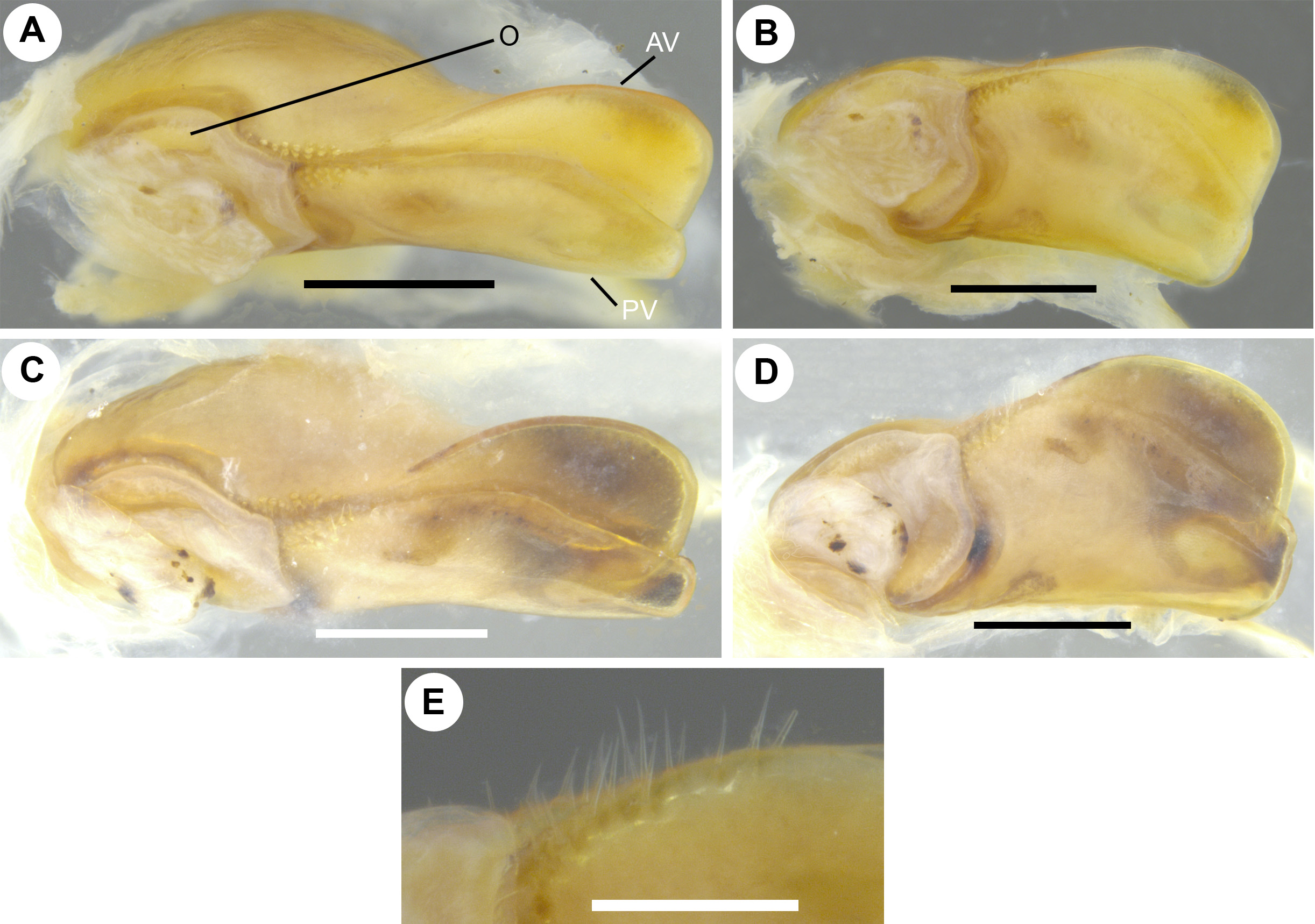
Female copulatory organ (vulva). Simple, bivalve-like ( Fig. 7A, C View FIGURE 7 ), consisting of two simple, subequally-sized, sclerotised valves ( Fig. 7 View FIGURE 7 A–D; AV, PV). Each valve proximo-laterally bearing 2 or 3 rows of short setae located towards opening ( Fig. 7E View FIGURE 7 ). ‘Operculum’ membranous, roughly circular with an anterior projection ( Fig. 7 View FIGURE 7 A–D; O).
Variations. Males (n=8): 47–50 body rings; length 74–76 mm; width 5.1–5.4 mm; length of midbody legs 4.71–4.9 mm. Females (n=16, excluding the subadult one): 49–50 body rings; length 75–76 mm; width 5.6–5.8 mm; length of midbody legs 4.81–4.92.
Distribution. Currently known only from India (Andhra Pradesh, Kerala, Maharashtra, Tamil Nadu, West Bengal) and Sri Lanka (Colombo, Kandy, Maha Iluppalama) ( Fig. 9 View FIGURE 9 ). Introduced populations are recorded in Australia ( Newport 1844a, 1844b) and Europe (Dublin, Ireland) ( Barber 2015). The record of X. carnifex in Borneo (Matang) ( Tömösváry 1885) is uncertain ( Pocock 1892). Records of X. carnifex in the U.S.A. ( Georgia) ( Koch 1847, 1863) are certainly wrong, even though Koch (1863) illustrated the species correctly ( Pocock 1892; Golovatch & Wesener 2016).
Natural history. Xenobolus carnifex is a synanthropic species and is mostly prevalent during the rainy season (June-July) ( Bhakat 2014; Sankaran, pers. obs.). It can be seen in open soils rich in organic matter, on fallen logs, on damp bricks and stones, as well as on wall surfaces and trunks of trees, all covered with bryophytes and fungi ( Bhakat 2014; Sankaran, pers. obs.). Rarely the species can be observed resting on the branches of shrubs (Sankaran, pers. obs.). During the rainy season, large numbers of individuals invade inside and on the roofs of buildings, thus becoming a nuisance pest ( Bai & Indra 1997; Alagesan & Muthukrishnan 2005b; Sankaran, pers. obs.).
Justification of the synonymy of X. acuticonus: Attems (1936) described X. acuticonus from Madras. It was characterised by a black body colouration, coupled with a row of hour-glass-shaped reddish spots on the dorsum, each male coxa 3 has a long process, an inverted V-shaped sternite of the anterior gonopods possesses a median spatula-shaped lamella, the anterior gonopod is with a broad coxite showing a short thumb-like mesal process, the telopodite medio-laterally has a globular process, the retrorse process of the telopodite features a downwards oriented flat and an antero-mesally oriented, finger-shaped process, the mesal branch of the posterior gonopod is with lateral ridges and a mesal hook and the main branch of the posterior gonopod has an apical fringe and a short, claw-shaped, lateral extension ( Attems 1936: fig. 87a–d). All these features are actually characteristic of X. carnifex (see Carl 1919: figs 25, 28–31; Wesener & Enghoff 2009: Fig. 25A–C). The topotype female genitalia of X. acuticonus match exactly in their structural details the female genitalia of X. carnifex (compare Fig. 7C View FIGURE 7 with Wesener & Enghoff 2009: fig. 23D and herein Fig. 7A View FIGURE 7 ). The generic distance analysis revealed that X. acuticonus differs from X. carnifex by a p-distance of 0.02% or mutations at 16 basepair positions ( Table 1). All these indicate, in agreement with Hoffman (1962), that in fact both X. carnifex and X. acuticonus belong to the same taxon. The species X. acuticonus is thus to be regarded as a junior synonym of X. carnifex .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Trigoniulidea |
|
Family |
|
|
Genus |
Xenobolus carnifex ( Fabricius, 1775 )
| Sankaran, Pradeep M. & Sebastian, Pothalil A. 2020 |
Erythroprosopon phoenix
| Moritz, M. & Fischer, S. - C. 1975: 251 |
Xenobolus acuticonus
| Golovatch, S. I. & Wesener, T. 2016: 34 |
| Basil-Rose, S. M. R. & Ravindranath, M. H. & Mercy, S. P. D. 2014: 331 |
| Jeekel, C. A. W. 2001: 54 |
| Bai, M. M. & Indra, T. J. 1997: 231 |
| Achar, K. P. 1987: 149 |
| Rangaswamy, H. R. & Devaiah, M. C. & Govindan, R. & Tippeswamy, C. 1983: 8 |
| Bano, K. 1983: 170 |
| Janardanan, K. P. & Ramachandran, P. 1982: 239 |
| Chowdaiah, B. N. & Kanaka, R. 1979: 17 |
| Sharma, G. P. & Handa, S. M. 1974: 678 |
| Rajulu, S. G. 1970: 143 |
| Nagarbhushanam, R. & Gokhale, K. S. 1968: 133 |
| Hoffman, R. L. 1962: 780 |
| Natarajan, R. 1959: 92 |
Erythroprosopon phoenix
| Verhoeff, K. W. 1936: 505 |
Xenobolus acuticonus Attems, 1936: 303
| Attems, C. 1936: 303 |
Xenobolus carnifex
| De Zoysa, H. K. S. & Nguyen, D. A. & Wickramasinghe, S. 2016: 457 |
| Golovatch, S. I. & Wesener, T. 2016: 34 |
| Barber, A. D. 2015: 52 |
| Bhakat, S. 2014: 185 |
| Alagesan, P. & Ramanathan, B. 2013: 1 |
| Karthigeyan, M. & Alagesan, P. 2011: 62 |
| Wesener, T. & Enghoff, H. & Wagele, J. - W. 2008: 38 |
| Alagesan, P. & Muthukrishnan, J. 2003: 313 |
| Jeekel, C. A. W. 2001: 54 |
| Valli, P. 1994: 322 |
| Alagesan, P. & Vanithapriya, S. 1992: 299 |
| Achar, K. P. 1987: 149 |
| Bano, K. 1983: 170 |
| Rajulu, S. G. 1970: 143 |
| Majumdar, G. 1967: 109 |
| Hoffman, R. L. 1962: 780 |
| Mukherjee, M. C. 1962: 25 |
| Ganapati, P. N. & Narasimhamurti, C. C. 1960: 581 |
| Attems, C. 1936: 304 |
| Carl, J. 1919: 394 |
Diaphoropus carnifex
| Silvestri, F. 1897: 651 |
Trigoniulus carnifex
| Silvestri, F. 1896: 28 |
Spirobolus carnifex
| Pocock, R. I. 1892: 166 |
| Daday, J. 1889: 129 |
| Tomosvary, E. 1885: 69 |
| Preudhomme de Borre, A. 1884: 73 |
| Karsch, F. 1881: 79 |
Iulus ruficollis
| Gervais, P. 1847: 175 |
Spirobolus
| Koch, C. L. 1847: 102 |
Spirobolus ruficollis
| Newport, G. 1844: 269 |
Spirobolus ruficollis
| Attems, C. 1914: 355 |
| Preudhomme de Borre, A. 1884: 76 |
| Newport, G. 1844: 13 |
Julus (Spirobolus) carnifex
| Brandt, J. F. 1841: 368 |
Iulus (sic!) carnifex
| Lucas, H. 1842: 532 |
| Gervais, P. 1837: 13 |
Julus
| Linnaeus, C. 1788: 3019 |
Iulus (sic!) carnifex
| Fabricius, J. C. 1781: 530 |
Julus carnifex
| Humbert, A. 1865: 57 |
| Brandt, J. F. 1833: 205 |
| Latreille, P. A. 1802: 76 |
| Olivier, A. G. 1792: 416 |
| Fabricius, J. C. 1781: 530 |
Iulus
| Fabricius, I. C. 1775: 428 |