Naineris lanai, Álvarez & Budaeva, 2023
|
publication ID |
https://doi.org/10.11646/zootaxa.5375.3.3 |
|
publication LSID |
lsid:zoobank.org:pub:4EBF95D8-B03D-4859-B127-AD0840DA10FB |
|
DOI |
https://doi.org/10.5281/zenodo.10248551 |
|
persistent identifier |
https://treatment.plazi.org/id/B28D373F-9ECC-4D80-AD0F-C7E1FFAA1CAA |
|
taxon LSID |
lsid:zoobank.org:act:B28D373F-9ECC-4D80-AD0F-C7E1FFAA1CAA |
|
treatment provided by |
Plazi |
|
scientific name |
Naineris lanai |
| status |
sp. nov. |
Naineris lanai View in CoL sp. n.
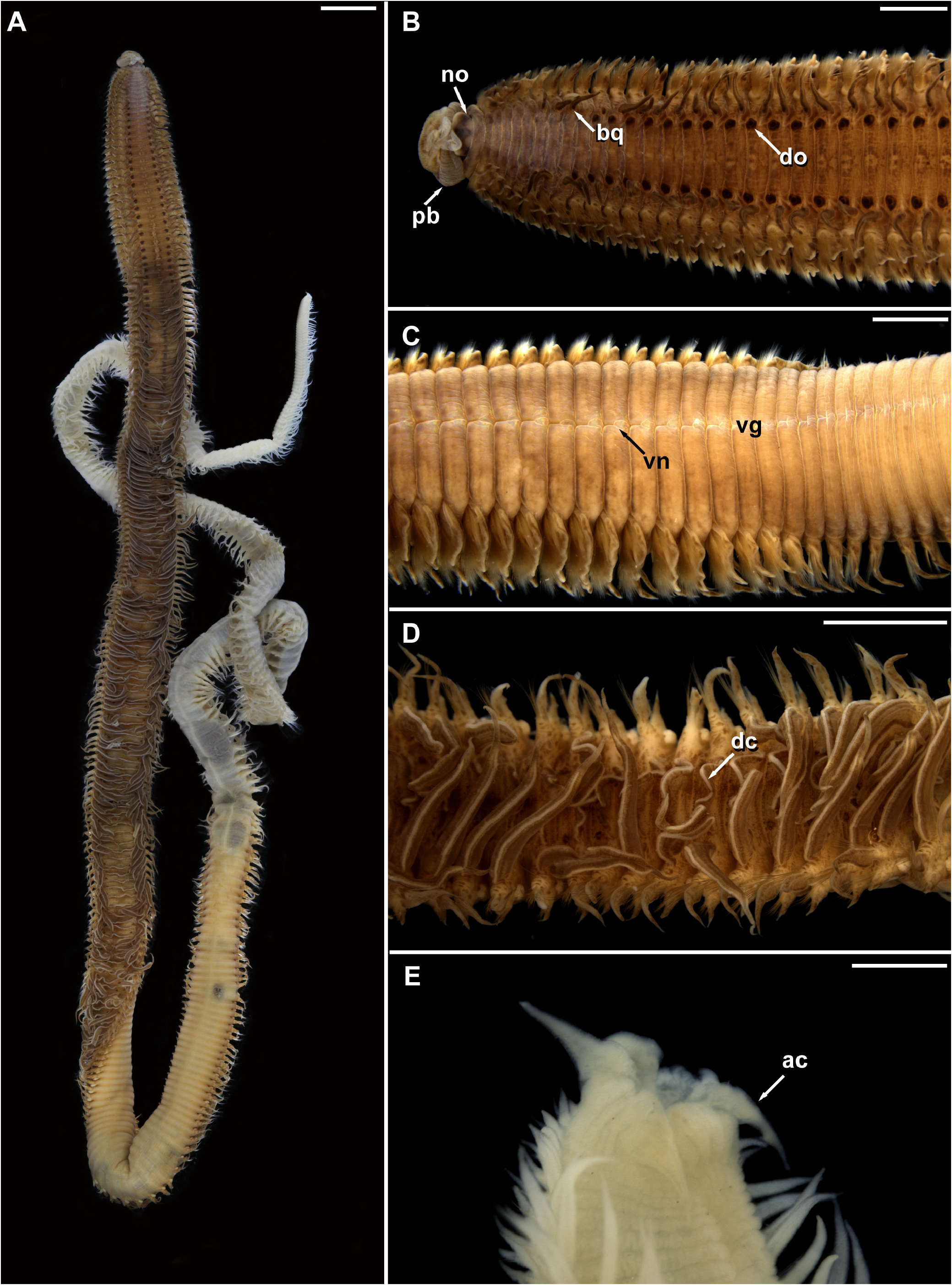
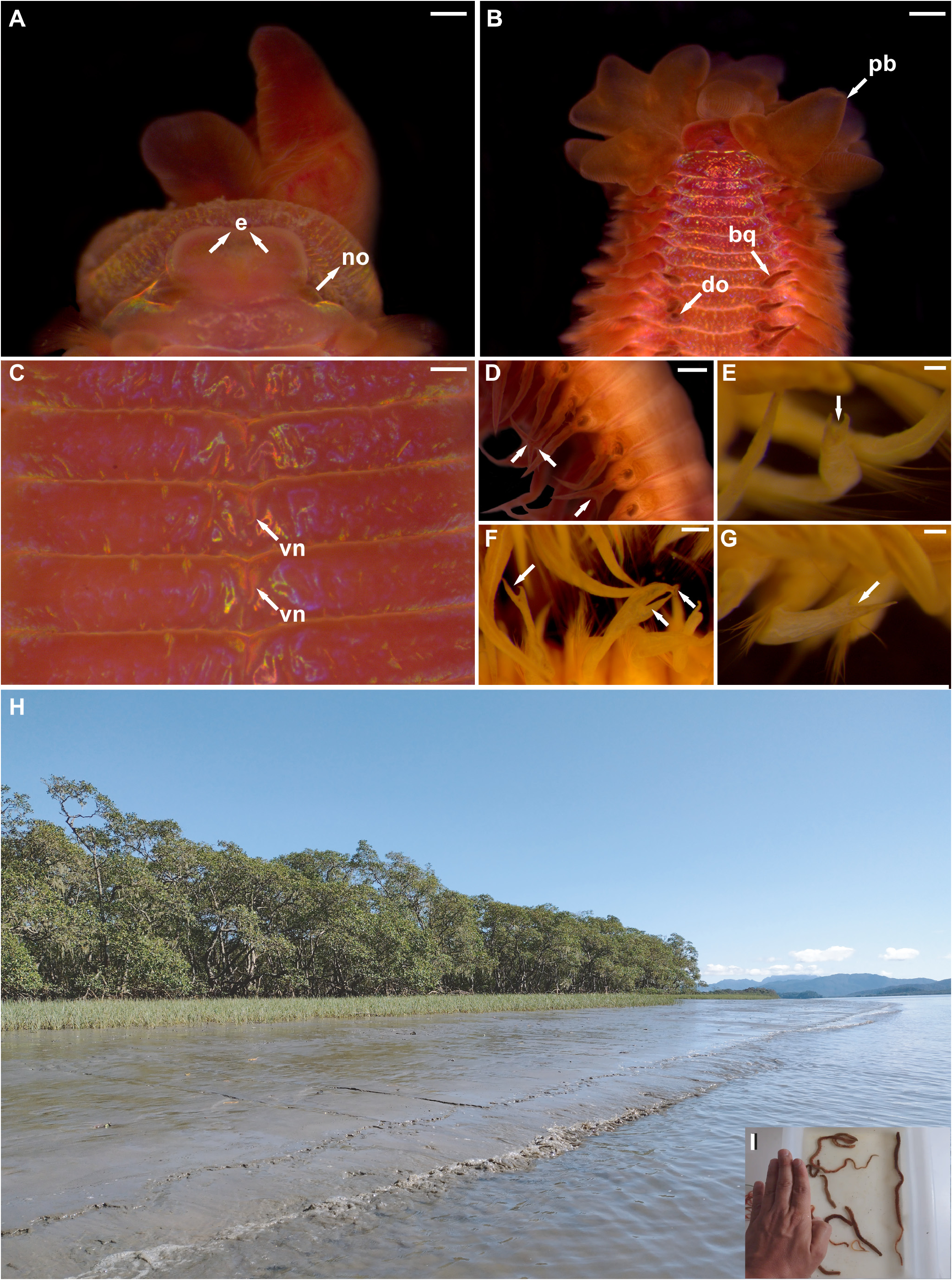
( Figs 9–11 View FIGURE 9 View FIGURE 10 View FIGURE 11 )
Material examined. Brazil. Paraná, Pinheiros Bay: MNRJP 007618 ( holotype) , MNRJP 007619 ( 2 paratypes) , MNRJP 007624 ( 1 paratype) , MNRJP 007622 ( 7 paratypes) , MNRJP 007621 ( 10 paratypes) , MNRJP 007634 ( paratype, DNA voucher Ns06) , MNRJP 007635 ( paratype, DNA voucher Ns07) , MNRJP 007636 ( paratype, DNA voucher Ns08) , MNRJP 007637 ( paratype, DNA voucher Ns09) , MNRJP 007638 ( paratype, DNA voucher Ns10) , MNRJP 007620 ( 6 paratypes) , Paranaguá Bay: MNRJP 007623 ( 2 paratypes) . Rio de Janeiro, Itacuruçá: MNRJP 007655 ( paratype, DNA voucher Ns36) , MNRJP 007656 ( paratype, DNA voucher Ns38) . São Paulo, Araçá: MNRJP 007654 ( paratype, DNA voucher Ns28) , Ilhabela, ZUEC-POL 16999 (2 spms) , ZUEC-POL 2756 (1 spm) , Caraguatatuba, ZUEC-POL 3780 (1 spm) , ZUEC-POL 3784 (1 spm) , ZUEC-POL 3786 (1 spm) , ZUEC-POL 3771 (1 spm) . Sergipe. Praia do Saco: MNRJP 1909 (1 spm).
Type locality: Brazil: Paraná ( Pinheiros Bay ), 25.409° S, 48.251° W, intertidal, 20 cm depth, anoxic mud, close to Spartina sp GoogleMaps .
Etymology. Naineris lanai is named in honor of R.A.’s late Ph.D. supervisor, the Brazilian scientist Paulo da Cunha Lana, in recognition of his substantial contribution to marine science and for believing in people.
Diagnosis. Thoracic neurochaetae including crenulated capillaries only; thoracic segments with ventral groove and notches; paired dorsal sensory organs present; ciliary dorsal crest in abdominal segments folded and hypertrophied; thoracic neuropodial lobes flattened and folded with irregular boundaries, with upper papilla; notopodial lobes undivided or forked.
Description. Large species ( Figs 9A View FIGURE 9 ; 11E View FIGURE 11 ), holotype complete (MNRJP 007618), 263 mm long, 5 mm wide, consisting of 431 chaetigers. Color in alcohol dark brown, with dark segmental spots; live specimens dark brown to reddish, with dark pigmented branchiae and dorsal organs, and long fluorescent cilia along axis; eyespots and nuchal organs yellowish, dorsal crest bearing long fluorescent cilia. Body separated into two distinct regions of approximately same width ( Fig. 9A View FIGURE 9 ); thorax and abdomen, with parapodia displaced dorsally in abdomen. Ventral surface of body rough, with prominent mid-ventral groove along most of body ( Figs 9C View FIGURE 9 ; 11C View FIGURE 11 ), well-marked in thoracic segments, represented by longitudinal notch on each segment, almost reaching consecutive ring ( Fig. 9C View FIGURE 9 ).
Prostomium wide, nearly square, spatulate in front ( Fig. 9B View FIGURE 9 ); eyespots present on lateral margins of prostomium, organized in two groups; nuchal organs present, as two lateral notches between prostomium and peristomium ( Fig. 9B View FIGURE 9 ), more conspicuous and globular in live specimens ( Fig. 11A View FIGURE 11 ). Peristomium as single achaetous ring; mouth opening located ventrally; proboscis wide, thick, bearing triangular lobes ( Figs 9B View FIGURE 9 ; 11A, B View FIGURE 11 ), densely ciliated ( Fig. 10A View FIGURE 10 ).
Branchiae from chaetigers 5–6, continuing along entire body ( Fig. 9A, B View FIGURE 9 ); elongate from first pair, widest basally, tapering to narrow apex, with medial and lateral cilia. Thoracic branchiae ⅓ of longest abdominal branchiae. Paired dorsal sensory organs from mid-thoracic chaetigers, anterior and medial to branchial bases, oval-shaped, clearly raised ( Figs 9B View FIGURE 9 ; 10B View FIGURE 10 ). Dorsal crest present in abdominal segments; straight at first, best developed and more extended in mid-abdominal segments becoming folded, as long as or even exceeding basal width of branchiae ( Figs 9A, D View FIGURE 9 ; 10D View FIGURE 10 ).
Thorax with 30 chaetigers ( 28–30 in paratypes), flattened. Parapodia biramous; interramal papilla absent. Thoracic notopodia with lanceolate lobes, more elongate in posterior chaetigers; forked or undivided. Neuropodial lobes wide, flat, with rough flanges with irregular borders, almost folded ( Fig. 9C View FIGURE 9 ). Abdominal notopodial and neuropodial lobes similar in shape, triangular to lanceolate, with thin apex; notopodial lobes more prolonged than neuropodial lobes ( Fig. 11D View FIGURE 11 ).
Thoracic notochaetae with two bundles of around 30–50 crenulated capillaries. Abdominal notochaetae with 15–20 crenulated capillaries in two bundles and furcate chaetae in lower position ( Fig. 10C View FIGURE 10 ); each furcate chaeta with unequal tines, bearing tiny needles; shaft with small barbs. Thoracic neurochaetae with about 7–9 rows of capillaries separated into two groups by oblique gap. Abdominal neurochaetae with 15–20 capillaries and 2–3 acicular spines.
Pygidium with terminal anus, bearing four anal cirri with forked or undivided tips, and rounded bases ( Fig. 9E View FIGURE 9 ).
Remarks. Naineris lanai sp. n. is similar to N. setosa s. str. in having only crenulated capillaries in thoracic neuropodia, dorsal crest, and branchiae from chaetigers 5–6. It differs from N. setosa in the dorsal crest being long and folded, and having marked ventral groove in thoracic segments, flat and folded thoracic neuropodial lobes, and often divided abdominal notopodial lobes.
Considering the anoxic habitat of the species, the hypertrophied ciliary dorsal crest, and parapodial lobes, are probably an adaptation to this environment. Similar correlation was established for some species of Nereididae and Opheliidae living in anoxic conditions ( Glasby et al. 2021). The shape of thoracic neuropodial lobes differs significantly in both species. In Naineris setosa s. str., neuropodial lobes are thick, elongated processes with rounded boundaries. In N. lanai sp. n., neuropodial lobes are enlarged, markedly flat, and folded with irregular boundaries. This may also be an adaptation to increase the surface area for oxygen uptake, such as in other polychaetes ( Hartman 1951; Nonato et al. 1986; Radashevsky & Lana 2009; Glasby et al. 2021).
Habitat. Large mature adults were sampled in black anoxic mud near Spartina sp. and mangroves from Pinheiros Bay, Paranaguá Estuarine Complex ( Fig. 11E View FIGURE 11 ). Juveniles were sampled in mud from São Paulo and fouling communities from Rio de Janeiro.
Distribution. South and Southeastern Brazil, from Paraná to Rio de Janeiro, intertidal.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Sedentaria |
|
Order |
|
|
Family |
|
|
Genus |