Chaetozone artaspinosa, Blake, 2022
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5113.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:EB01C862-025E-493F-8CA9-934B4F1626AF |
|
DOI |
https://doi.org/10.5281/zenodo.6958024 |
|
persistent identifier |
https://treatment.plazi.org/id/054C717B-7132-234C-65DD-FA01FCBBF822 |
|
treatment provided by |
Plazi |
|
scientific name |
Chaetozone artaspinosa |
| status |
sp. nov. |
Chaetozone artaspinosa new species
Figures 20–23 View FIGURE 20 View FIGURE 21 View FIGURE 22 View FIGURE 23
Table 4 View TABLE 4
urn:lsid:zoobank.org:act:27DAFA43-5EED-4DCD-A274-D2D73280CB40
Chaetozone vivipara: Hilbig et al. 1996: 24 View in CoL ; 29–30, 60, 63, D-1; Blake et al. 1998a: 77, E-1; Maciolek et al. 2006: C-24; 2008: 4-23–4-25, 4-28. Not Christie 1984.
Material examined. (384 specimens) Northeastern USA, Boston Harbor Massachusetts, MWRA Harbor Monitoring Program, Sta. T-05A: Rep 2, 25 Apr 2002, 70.960617°N, 42.339718°W, 9.0 m, holotype ( MCZ 161934 View Materials ) GoogleMaps , 62 paratypes ( MCZ 161935 View Materials ) ; 01 Aug 2007, Rep. 1, 70.9607315°N, 42.3396835°W, 16.2 m, 1 specimen on SEM stub ( MCZ 161936 View Materials ) GoogleMaps , 3 specimens on SEM stub ( MCZ 161937 View Materials ) . Sta. T-01: Rep. 2, Apr 1995, 42°20.95 ′ N, 70°57.81 ′ W, 4 m (66 juveniles, MCZ 161938 View Materials ) GoogleMaps ; Rep. 4, 22 May 2002, 70.963600°N, 42.349217°W, 4.0 m, 15 paratypes ( MCZ 161939 View Materials ) GoogleMaps . Sta. T-02: Rep. 2, Apr 1995, 42°20.57 ′ N, 71°00.12 ′ W, 9 m (75 juveniles, MCZ 161940 View Materials ) GoogleMaps ; Rep. 2, 05 Aug 2009, 71.0019302°N, 42.3429679°W, 7.5 m, 10 paratypes ( MCZ 161941 View Materials ) GoogleMaps . Sta. T-03: Rep. 2, Apr 1995, 42.330°N 70.962°W, 8.7 m (140, MCZ 161943 View Materials ) GoogleMaps ; Sta. T-07: Rep. 2, 25 Apr 2002, 70.978615°N, 42.289318°W, 7.5 m, 11 paratypes ( MCZ 161942 View Materials ) GoogleMaps .
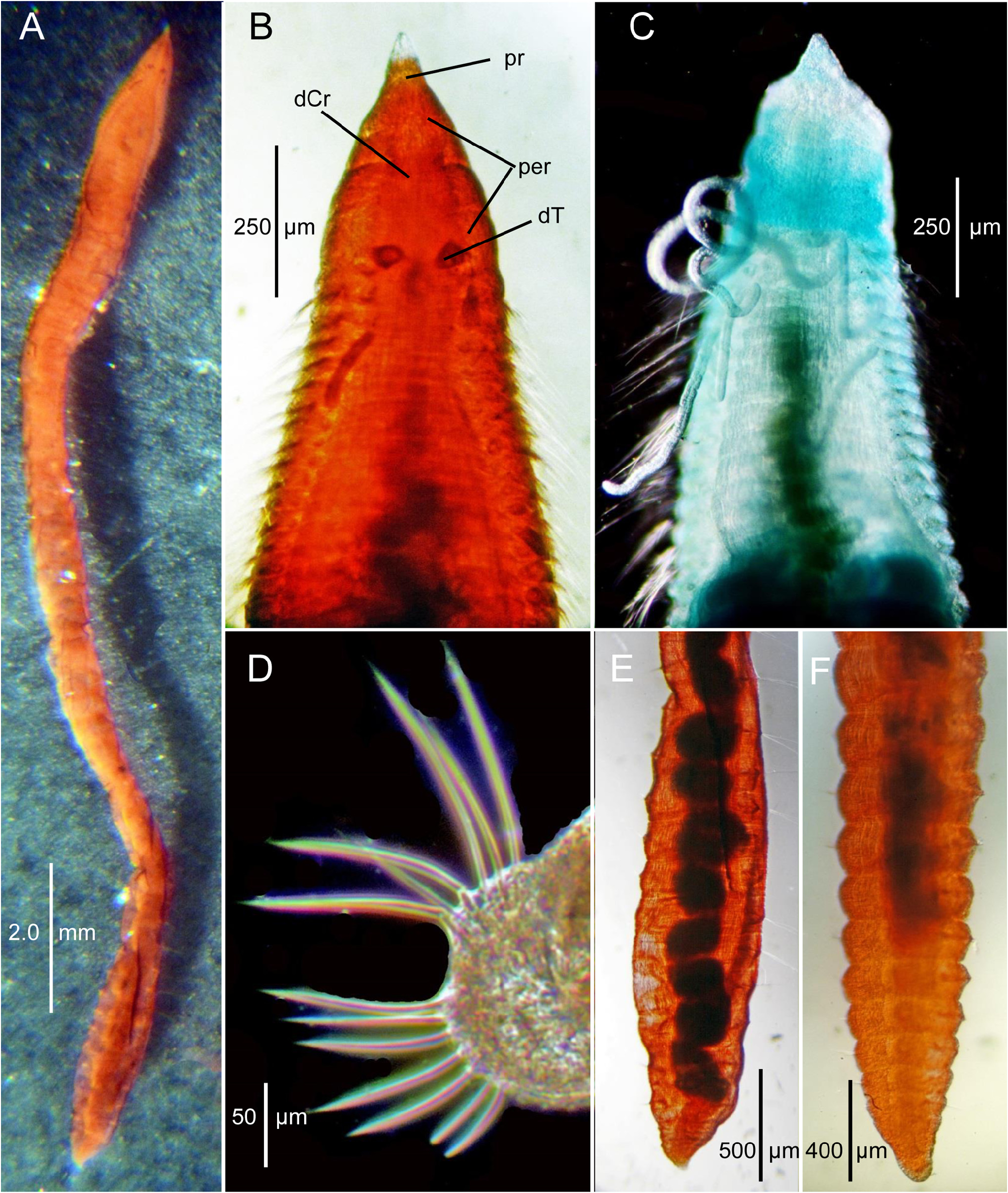
Description. A small to moderately sized species, holotype complete, with about 80 setigers, 11.2 mm long and 0.54 mm wide anteriorly ( Fig. 21A View FIGURE 21 ); large paratype with 102 setigers, 14.4 mm long, and 0.44 mm wide ( Fig. 20A View FIGURE 20 ); a small paratype (MCZ 161935) with 44 setigers, 3.3 mm long, and 0.73 mm wide across anterior segments. Body elongate, narrow, only weakly expanded anteriorly, tapering to posterior end ( Fig 20A View FIGURE 20 ), sometimes weakly enlarged. Anterior setigers about ten times wider than long; middle segments up to five times wider than long. Dorsal surface rounded, slightly elevated above parapodia, without dorsal groove or ridge; ventral surface flattened with distinct ventral groove or channel extending from about setiger 1 along anterior and middle setigers, indistinct in posterior setigers. Posterior segments with parapodia elevated dorsally. Posterior end dorsoventrally flattened tapering to pygidium ( Figs. 20A, E–F View FIGURE 20 , 21B View FIGURE 21 ). Color in alcohol opaque white to light tan, with no pigmentation.
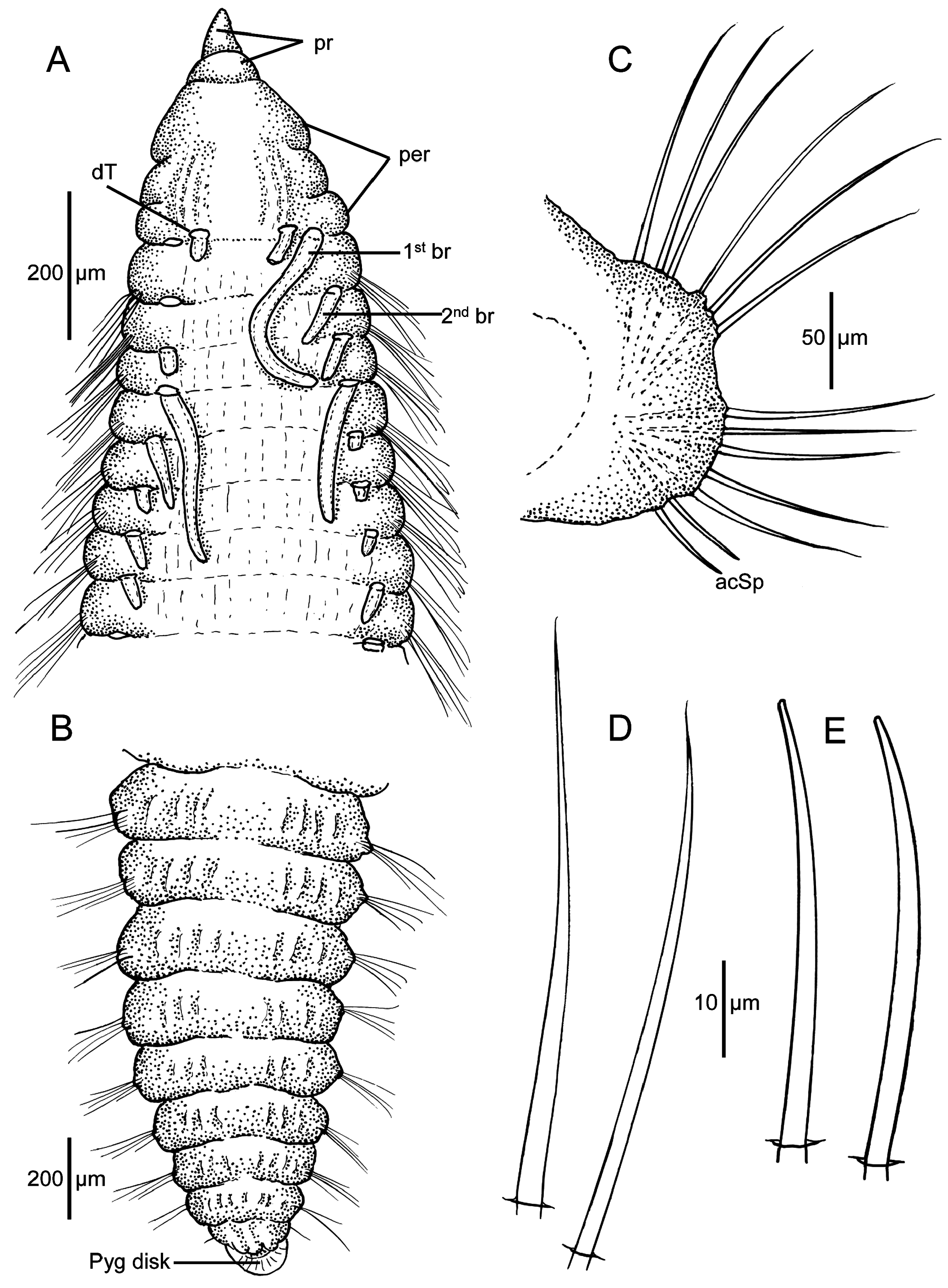
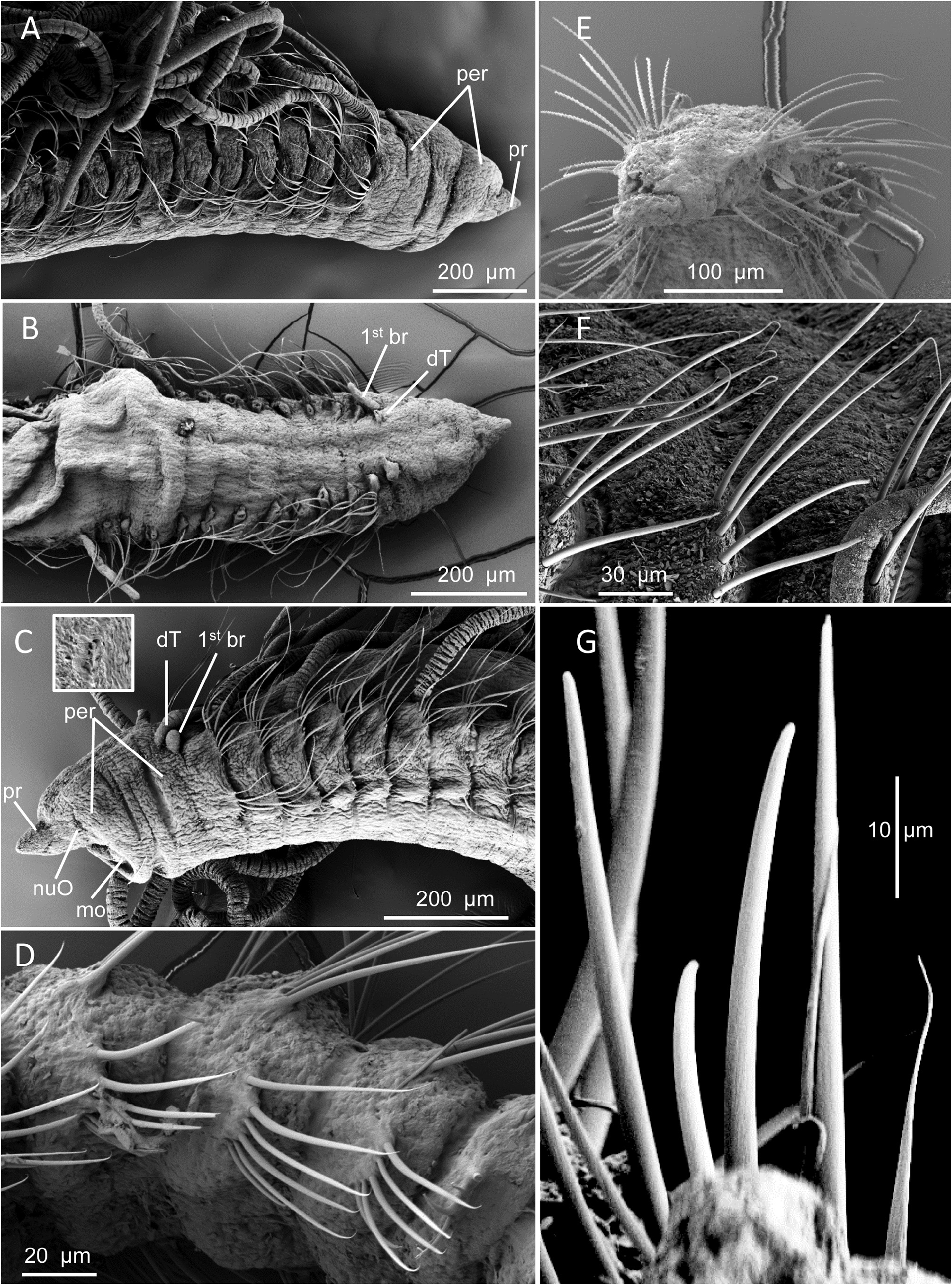
Pre-setiger region triangular in shape, slightly longer than wide ( Figs. 20B View FIGURE 20 , 21A View FIGURE 21 , 22B View FIGURE 22 ); about as long as first five setigers in smaller specimens and about first ten setigers in larger specimens; Prostomium short, triangular, tapering to narrow pointed tip ( Figs. 20A–B View FIGURE 20 , 21A View FIGURE 21 , 22A View FIGURE 22 ); eyespots absent; nuchal organs narrow oval-shaped ciliated openings on posterior lateral margins ( Fig. 22C View FIGURE 22 inset). Peristomium with two prominent lateral grooves producing three rings ( Figs. 20B View FIGURE 20 , 21A View FIGURE 21 , 22A–C View FIGURE 22 ), but these not crossing dorsal surface, weakly developed dorsal crest present ( Figs. 20B View FIGURE 20 , 21A View FIGURE 21 ); some specimens with longitudinal groove in crest producing two parallel crests ( Fig. 22B–C View FIGURE 22 ). Dorsal tentacles arise from medial location on posterior margin of peristomium ( Figs. 20B View FIGURE 20 , 21A View FIGURE 21 , 22B–C View FIGURE 22 ); first pair of branchiae lateral to tentacles; second pair of branchiae on setiger 1 dorsal to notosetae; subsequent branchiae in similar location ( Figs. 21A View FIGURE 21 , 22C View FIGURE 22 ). Branchiae present along most of body.
Parapodia of anterior segments short thickened vertical lobes from which setae arise; these lobes weakly swollen, with noto- and neurosetae arising directly from them. Parapodia of posterior segments thinner, narrower, bearing acicular spines and capillaries in spreading fascicles ( Figs. 20D View FIGURE 20 , 21C View FIGURE 21 , 22D, F View FIGURE 22 ).
All setae long, narrow. Anterior and middle setigers with setae all long, thin capillaries ( Fig. 21D View FIGURE 21 , 22F View FIGURE 22 ), with 8–10 in notopodia and 7–8 in neuropodia ( Fig. 22A–C View FIGURE 22 ); posterior setigers with 5–8 capillaries in notopodia ( Fig. 22F View FIGURE 22 ) and 4–5 capillaries and 1–3 acicular spines in neuropodia ( Fig. 21E View FIGURE 21 ). Spines first present in neuropodia from middle body segments: about setiger 55 in 80-setiger holotype, setiger 70 in large 102-setiger paratype, and setiger 35 in smaller 44-setiger paratype; spines not observed in notopodia. Individual spines long, narrow, straight or only weakly curved, not sigmoidally curved; terminating in bluntly rounded tip ( Figs. 21E View FIGURE 21 , 22G View FIGURE 22 ).
Pygidium a rounded disk ventral to anal opening ( Figs. 20E–F View FIGURE 20 , 21B View FIGURE 21 , 22E View FIGURE 22 ).
Methyl green staining. Peristomium retaining stain in irregular pattern ( Fig. 20C View FIGURE 20 ); tip of prostomium sometimes retaining stain, otherwise no pattern.
Remarks. Chaetozone artaspinosa n. sp. was first observed in Boston Harbor benthos in August 1995 when large numbers of a small unknown cirratulid appeared in benthic samples collected as part of the harbor-wide benthic monitoring survey. Some of these specimens were shown to the late Dr. Mary E. Petersen who was visiting our laboratory in Woods Hole at the time. Dr. Petersen indicated that she had collected similar-appearing specimens in Denmark and had identified them as Tharyx vivipara Christie, 1984 , an estuarine species from northeastern England in the U.K. that exhibited an unusual form of larval viviparity where early development occurred entirely within the bodies of females ( Christie 1984). Dr. Petersen was convinced that our Boston Harbor specimens were the same as the species from the U.K. and Denmark. She was also convinced that they belonged to the genus Chaetozone instead of Tharyx , despite the original description and illustrations of T. vivipara clearly indicating that all setae were pointed capillaries and that none of the Boston Harbor specimens exhibited any evidence of viviparity. Petersen (1999) subsequently referred T. vivipara to the genus Chaetozone as part of her review of cirratulid reproduction and development, but did not provide a redescription of the European species. Hartmann-Schröder (1996) had earlier referred T. vivipara to Aphelochaeta with a brief description indicating that all setae were capillaries. Various technical reports of cirratulids from the U.K. summarized by Worsford (2009) have referred T. vivipara to Chaetozone based largely on Petersen (1999) but with only minimal descriptive notes.
A review of Dr. Petersen’s unpublished notes, sketches, and prepared slides of Tharyx vivipara included information on the specimens she had collected from Limfjord, Denmark, in 1983. The notes and sketches appear to refer to the same species described by Christie (1984), but there is no evidence that she actually observed acicular spines on her specimens that would confirm referral of the species to Chaetozone . A detailed sketch of the anterior end in dorsal view was prepared, but none depicting setae. There are five well-prepared slides of posterior ends and a few separate parapodia of the Limfjord specimens. These are in good condition and clearly show spreading fascicles of noto- and neurosetae from pre-pygidial segments. Although some of the setae are broken, all intact setae are capillaries; no acicular spines are present among the intact capillaries. The longer capillaries have a dark core and it is possible that such setae might have been mistaken for spines. However, these slides do not support the transfer of Tharyx vivipara to Chaetozone . It would appear that at least for the Danish specimens, Dr. Petersen based her assumption of a Chaetozone identity on the overall shape of the body and posterior setigers as being similar in appearance to other Chaetozone species she had observed. Based on these observations and my interpretation of Dr. Petersen’s observations and records, T. vivipara should be included in the genus Aphelochaeta following Hartmann-Schröder (1996). However, it is possible that the specimens from Denmark and the U.K. are not the same species.
Despite the identity of the Boston Harbor specimens being based on anecdotal observations and comments by Dr. Petersen, they were subsequently referred to as Chaetozone vivipara in the database by the project team and have continued to be identified as such in subsequent monitoring surveys; there being no alternative identification available. Systematic studies on Cirratulidae by the author and supported by the NSF PEET program (2001–2008) included extensive studies on global Cirratulidae and included traditional methods as well as SEM to assist in defining the morphology of local species. A recent review of specimens and SEM images of “ C. vivipara ” from Boston Harbor clearly demonstrates that they are not the same species described by Christie (1984), but another species having an entirely different morphology here described as Chaetozone artaspinosa n. sp.
Chaetozone artaspinosa n. sp. is most similar to C. castanea from off Peru and Chile in having acicular spines that are long, narrow, and straight or only weakly curved ( Blake 2018) rather than thick and sigmoidally curved. Chaetozone artaspinosa n. sp. differs from C. castanea in having three peristomial rings instead of four (with the first merged with the prostomium), no acicular notopodial spines instead of 2–3, 1–3 neuropodial acicular spines instead of 5–6 and a sub-anal pygidial lobe that is thick and cushion-like instead of disk-like. In addition, the body of C. castanea is heavily pigmented brown whereas, C. artaspinosa n. sp. has no pigmentation on the body.
Locally, Chaetozone artaspinosa n. sp. may be superficially mistaken for Tharyx acutus Webster & Benedict, 1887 , with which it may occur. Both species have acicular spines limited to the neuropodia. However, C. artaspinosa n. sp. has three distinct peristomial rings whereas T. acutus has an elongate smooth peristomium with only lateral grooves denoting the posterior lip of the mouth, but not producing separate rings. In addition, posterior segments of T. acutus are expanded, with a broad ventral groove into which the neurosetae project, including the acicular spines that have a sub-bidentate knobby tip. In contrast, the posterior segments of C. artaspinosa n. sp. are narrow, rounded, and while variously flattened ventrally, lack a distinct ventral groove; the neuroacicular spines are elongate and have a simple, narrowly blunted tip without any apical knobs or teeth.
Biology. Chaetozone artaspinosa n. sp., as C. vivipara , was first reported in the August 1995 MWRA Boston Harbor collections ( Hilbig et al. 1996) but was not reported in the spring (April) 1995 samples. In the August samples, the species was reported in densities of 8,000 to 29,000 individuals per m 2 at stations T-01 and T-02 (Deer Island Flats and off Logan Airport, northern Harbor). Hilbig et al. (1996) suggested that large numbers of unidentified juvenile cirratulids enumerated in the April 1995 samples from these same two stations might have belonged to this species; juvenile cirratulids were the third most abundant taxon at both stations in the April 1995 samples.
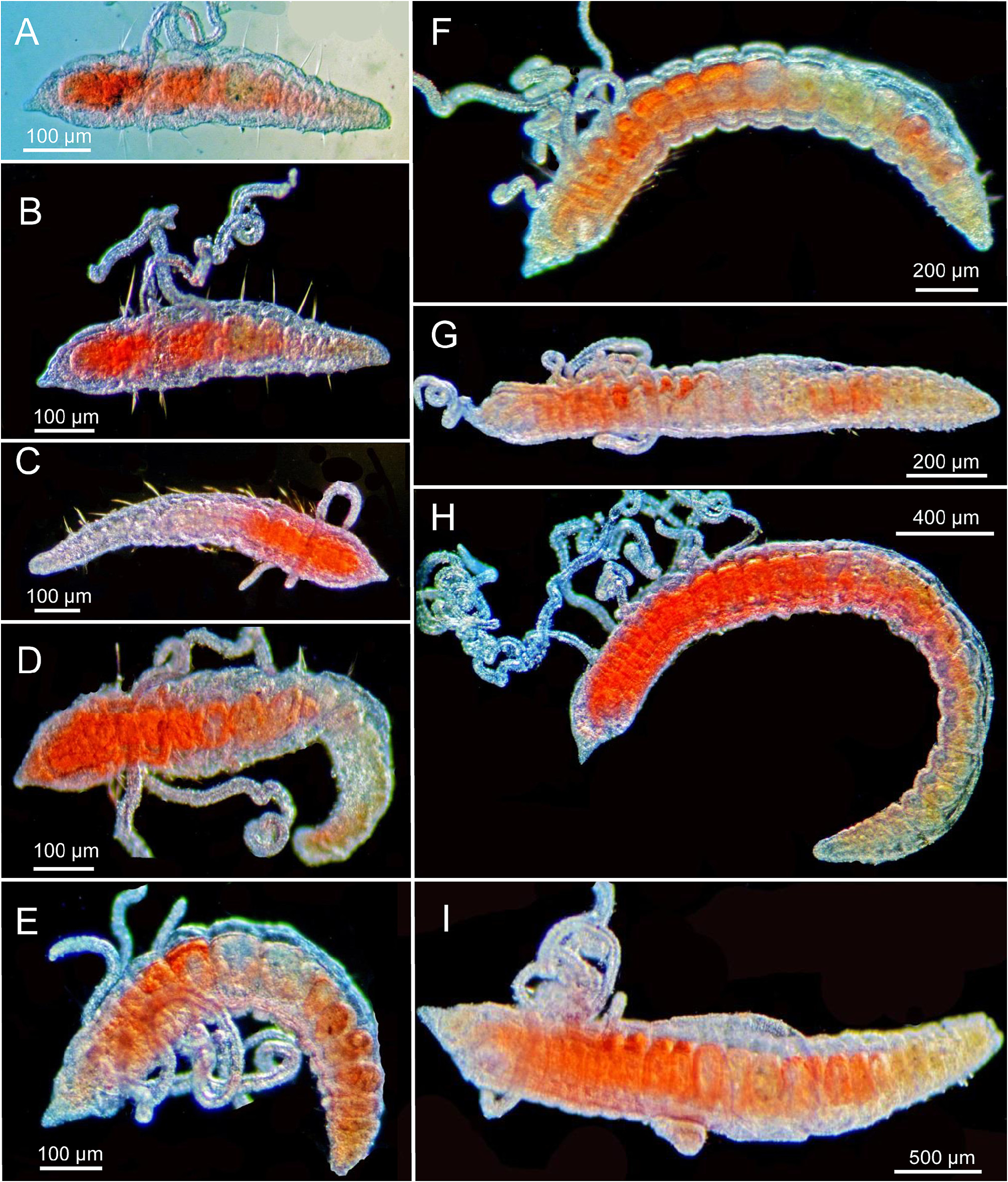
In order to determine if the juvenile cirratulids set aside in April of 1995 were C. artaspinosa n. sp., some of the specimens available from replicates from Stations T-01, T-02, and T-03 were examined and determined to indeed include juveniles of C. artaspinosa n. sp. In addition, adults packed with eggs were also present at Sta. T-03. Some of the meristic data is presented in Table 4 View TABLE 4 and images of juveniles from 10 to 28 setigers in length are illustrated in Figure 23 View FIGURE 23 . The smallest juveniles with 10 and 11 setigers were grub-like post-larval forms ( Fig. 23A–B View FIGURE 23 ) but had well-developed dorsal tentacles; one or two stiff capillary setae were present in noto- and neuropodia, but acicular spines were not present. Juveniles with 12 and 13 setigers ( Fig. 23C–E View FIGURE 23 ) exhibit some elongation of the trunk region and branchiae were present on a few anterior setigers; one acicular spine was observed on the tenth setiger of a 14-setiger specimen ( Table 4 View TABLE 4 ). Larger juveniles with 19 setigers ( Fig. 23F View FIGURE 23 ), 22 setigers ( Fig. 23G View FIGURE 23 ), 25 setigers ( Fig. 23H View FIGURE 23 ), and 28 setigers ( Fig. 23I View FIGURE 23 ) exhibit an elongation of the body from the thick and grub-like post-larval shape to an elongated body with a consistent width and length but no enlargement of anterior and posterior setigers as present in adults ( Fig. 20A View FIGURE 20 ). Acicular spines with a narrow rounded tip are consistently first present from about the 14- setiger stage. Initially, the spines are first present from setiger 10 in specimens with 14–20 setigers; specimens with 25–28 setigers have the spines first present at setigers 14–15. Thus, the juveniles have neuropodial acicular spines first present in middle body segments as with the much larger adults (holotype: first acicular spine in setiger 55 of 80). These observations of juveniles therefore suggest that with growth and setal replacement, acicular spines retain their position in the middle body segments.
Stations T-01, T-02, and T-05A are locations in Boston Harbor where Chaetozone artaspinosa n. sp. occurred consistently ( Maciolek et al 2006, 2008). The species was typically among the top 10 or 15 most abundant benthic invertebrates at these sites. These three sites are located in the northern part of Boston Harbor near the main shipping channel into Boston. Sediments at these sites have lower percentages of fines (silt + clay ca. 20%) and higher sand inventories. In addition, total organic carbon is relatively low at about 0.8% ( Maciolek et al 2008).
Several specimens of C. artaspinosa n. sp. collected in April 1995 (Sta. T-03) and 2002 (Sta. T-05A) were observed with the middle segments packed with eggs measuring up to 95–125 µm in diameter. Gametes were not observed in specimens from the August surveys, suggesting that this species reproduces in the spring. The appearance of juveniles in April samples previously discussed supports this suggestion.
Etymology. The epithet is from artus, Latin for narrow and spina, Latin for thorn, referring to the narrow acicular spines that characterize this species.
Distribution. Known only from Boston Harbor, Massachusetts, in shallow subtidal sediments from 4–16 m, but likely widespread in estuaries along the U.S. northeastern coast.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Chaetozone artaspinosa
| Blake, James A. 2022 |
Chaetozone vivipara:
| Blake J. A. & Maciolek N. J. & Rhoads D. C. & Gallagher E. D. & Williams I. P. 1998: 77 |
| Hilbig, B. & Blake, J. A. & Rhoads, D. C. & Williams, I. P. 1996: 24 |