Ctenodrilus pacificus, Magalhães, Wagner F., Weidhase, Michael, Schulze, Anja & Bailey-Brock, Julie H., 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4103.4.2 |
|
publication LSID |
lsid:zoobank.org:pub:3EAC4E08-6076-4A53-BE0B-05947D3444C5 |
|
DOI |
https://doi.org/10.5281/zenodo.5612342 |
|
persistent identifier |
https://treatment.plazi.org/id/1C528797-FF91-FFB6-6AA4-958E84CC2FB5 |
|
treatment provided by |
Plazi |
|
scientific name |
Ctenodrilus pacificus |
| status |
|
Ctenodrilus pacificus sp. nov. Figures 2‒5 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5
Material examined. Holotype: Duke Kahanamoku Lagoon, south shore of Oahu, Hawaii, 21.282587, - 157.838919, on green algae, shallow subtidal, 29 Aug 2015, coll. W. Magalhães ( USNM 1297761). Paratypes same locality, date and collector as holotype (6, USNM 1297762; 5, BMNH 2016.360-364; 10, BMNH 2016.365- 374).
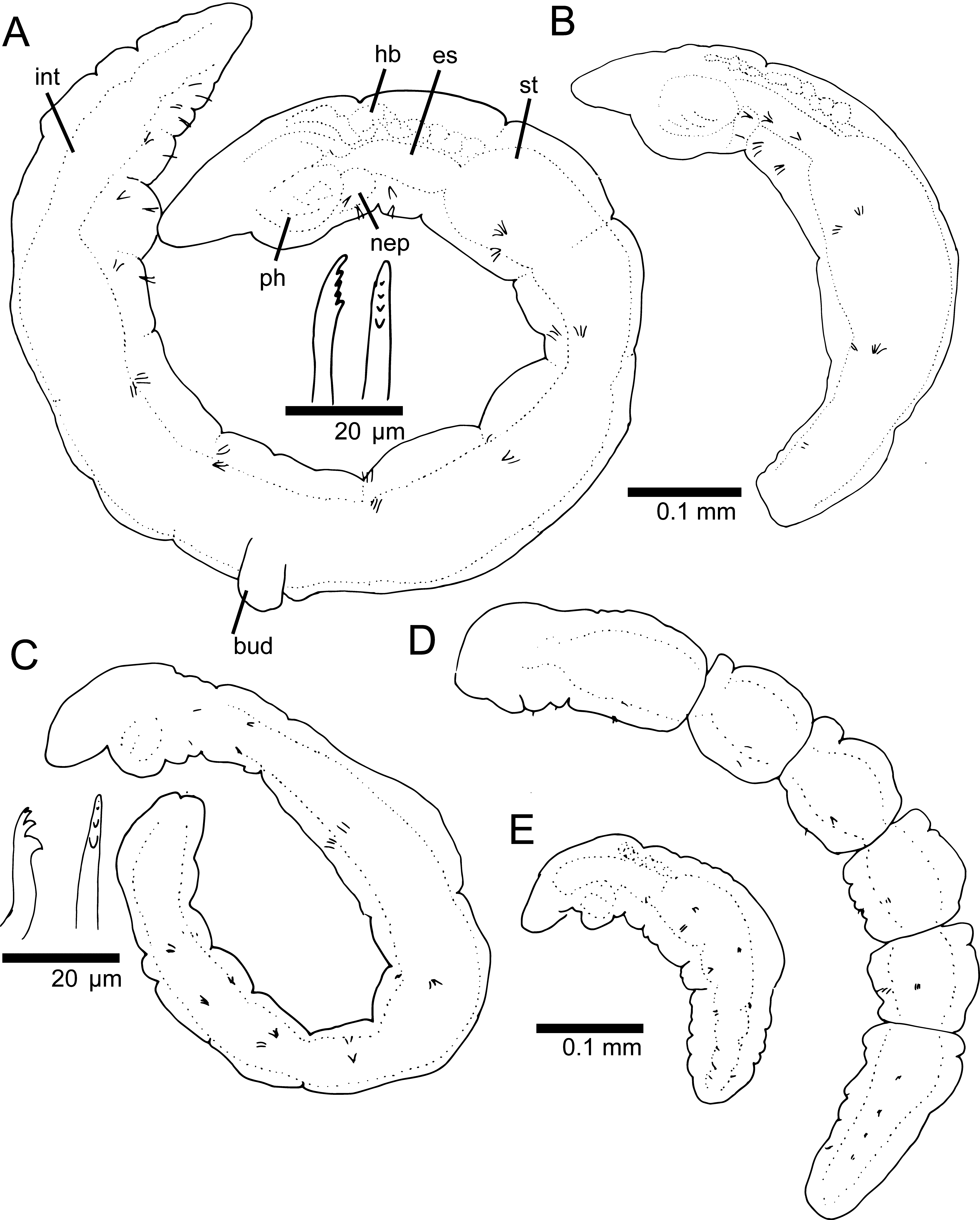
Comparative material examined: Ctenodrilus serratus : United States, Massachusetts, Nahant, Marine Science Institute seawater tables, coll. 0 6 Apr 1974, Robert E. Palmer (5 specimens, one in early stage of asexual budding, USNM 51480); United States, Massachusetts, Cuttyhunk Island, on sponges, coll. 0 1 Sept 1953, M. Pettibone, (5 badly preserved specimens, USNM 50886); United States, Massachusetts, Cuttyhunk Island, on Pecten irradians , coll. 18 Aug 1952, M. Pettibone, (3 badly preserved specimens, USNM 50889); United States, Louisiana, Gulf of Mexico, Bay Marchand Lease Area, 12 meters, 29.0472, -90.1628, CGPS Expedition, MMS Collections, Central Gulf Platform Study, coll. 19 Jun 1978, Mfj; Deh (1, USNM 67987); San Salvador, Bahamas (1, USNM 1044811); Belize (1, USNM 321390). Ctenodrilus paucidentatus : Holotype ( BMNH 1976.255, vial empty) and paratype (1, BNHM 1976.256), Dendropoma reef opposite Wadi Kabila, South Sinai, Gulf of Elat, 30th April, 1971, coll. Dr. M.N. Ben-Eliahu.
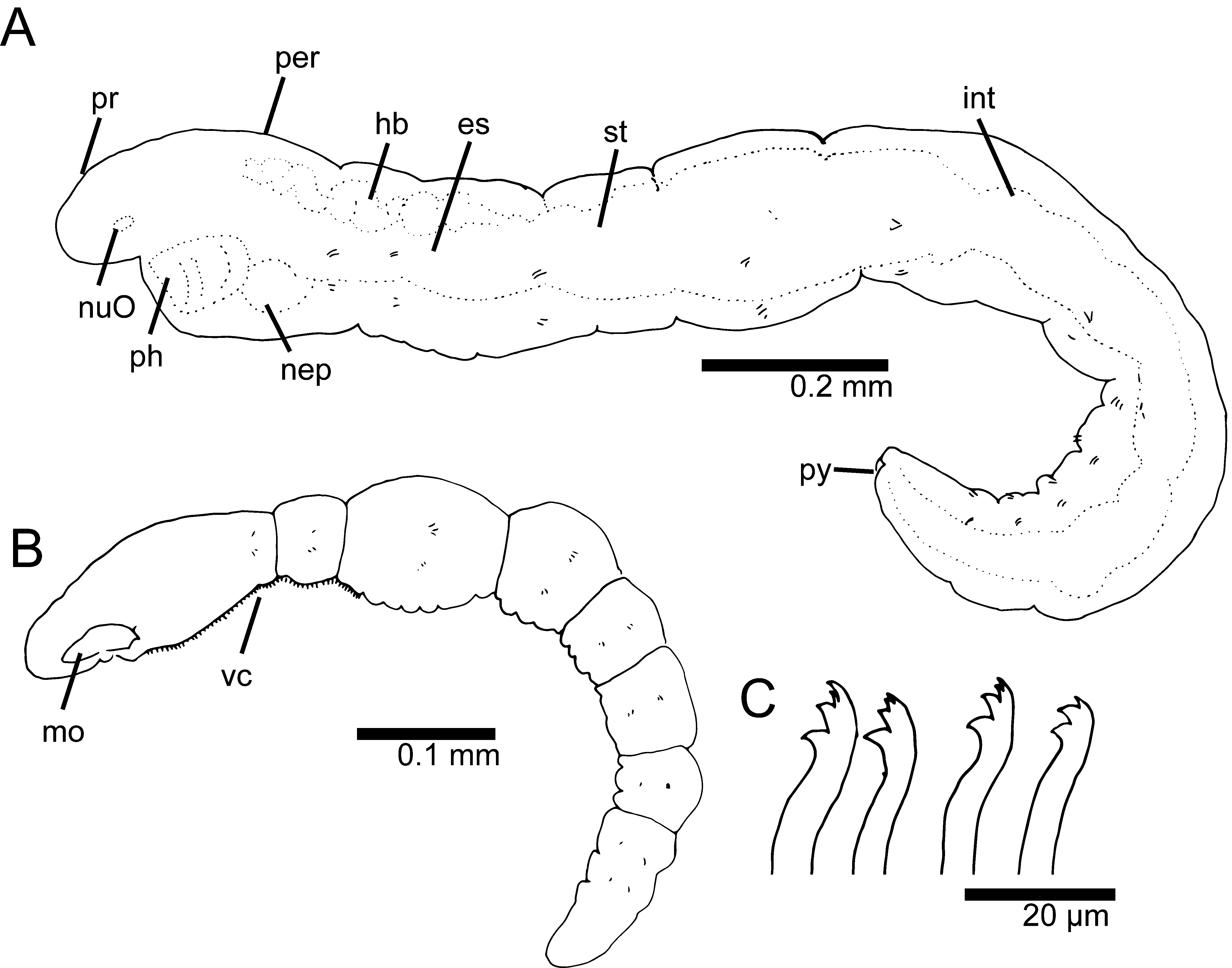
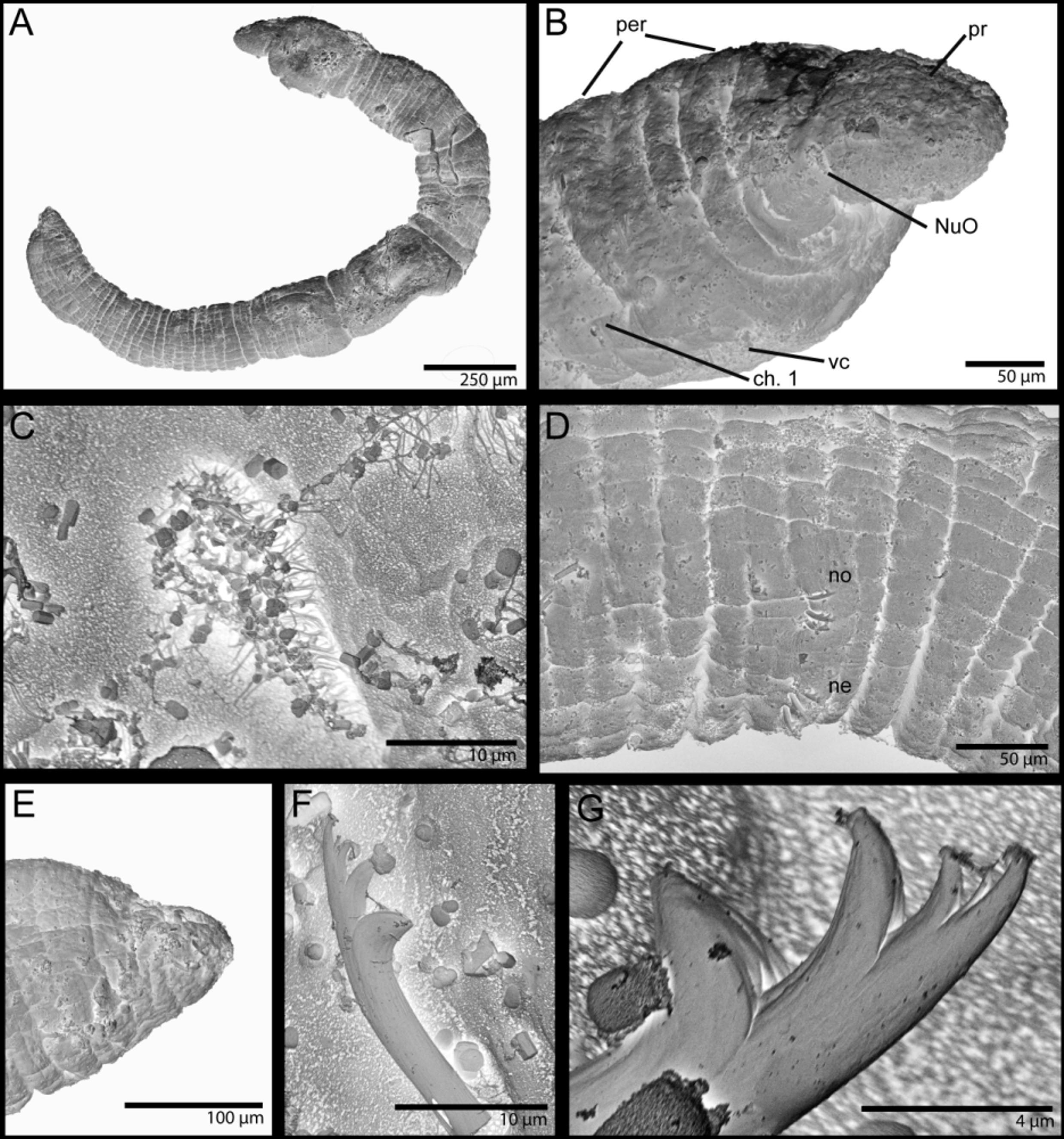
Description. Live specimens 1–3.5 mm long, 0.2 mm wide for up to 14 chaetigers; preserved specimens with shorter total length, up to 2.5 mm long. Holotype 1.8 mm long, 0.2 mm wide for 12 chaetigers. Paratypes up to 2 mm long and 13 chaetigers. Body short to elongate, rounded in cross section; first three segments as long as wide, segments becoming 2–3 times longer than wide from chaetiger 4; body slightly tapering posteriorly to elongate pygidial segment ( Figs 2 View FIGURE 2 A, 3A, 4A). Live specimens with black epidermal glands throughout the body ( Fig. 3 View FIGURE 3 ), uniformly distributed, yellow pigments also observed but scarce; preserved specimens lack pigmentation.
Prostomium short, broadly rounded with a pair of postero-lateral rounded nuchal organs ( Fig. 4 View FIGURE 4 A–C); eyespots absent; nuchal organs in live specimens visible as unpigmented area ( Fig. 3 View FIGURE 3 A, B). Nuchal organs rounded ciliary patches of about 25 µm diameter ( Fig. 4 View FIGURE 4 C). Anterior region (prostomium, peristomium and chaetiger 1) with indistinct separation dorsally and ventrally in live specimens; clear segment demarcation between chaetigers 1 and 2. SEM shows clear lateral separation between prostomium and peristomium and between peristomium and chaetiger 1; peristomium as single achaetous segment with lateral sub-annulation/wrinkles ( Fig. 4 View FIGURE 4 B). Fields of short cilia present on ventral region from posterior end of peristomium to anterior end of chaetiger 3 ( Fig. 2 View FIGURE 2 B); dorsal cilia present to end of chaetiger 2, cilia longer than ventral and as isolated tufts. Palps and branchiae absent. Digestive system starts with ventral and smooth muscular pharynx, lacking cilia; divided into three regions: esophagus narrow and continues to middle of chaetiger three with long cilia lining internal wall and no distinct coloration; straight and slightly inflated stomach region, reddish in color, cilia absent, stomach continues to end of chaetigers 6–8 in individuals with 10–13 chaetigers; continuing with convoluted intestine region, ciliated, lighter in color than stomach and ending in dorsal anal aperture; cilia from intestine walls not extending beyond anal aperture ( Figs 2 View FIGURE 2 A, 3A, C). Circulatory system with dorsal and ventral vessels, dorsal vessel enlarged as heart body from chaetiger 1 to middle of chaetiger 3 ( Figs 2 View FIGURE 2 A, 3B). A pair of nephridia as simple sacks present below muscular pharynx on peristomial segment, sometimes seen from anterior end of chaetiger 1 ( Fig. 2 View FIGURE 2 A).
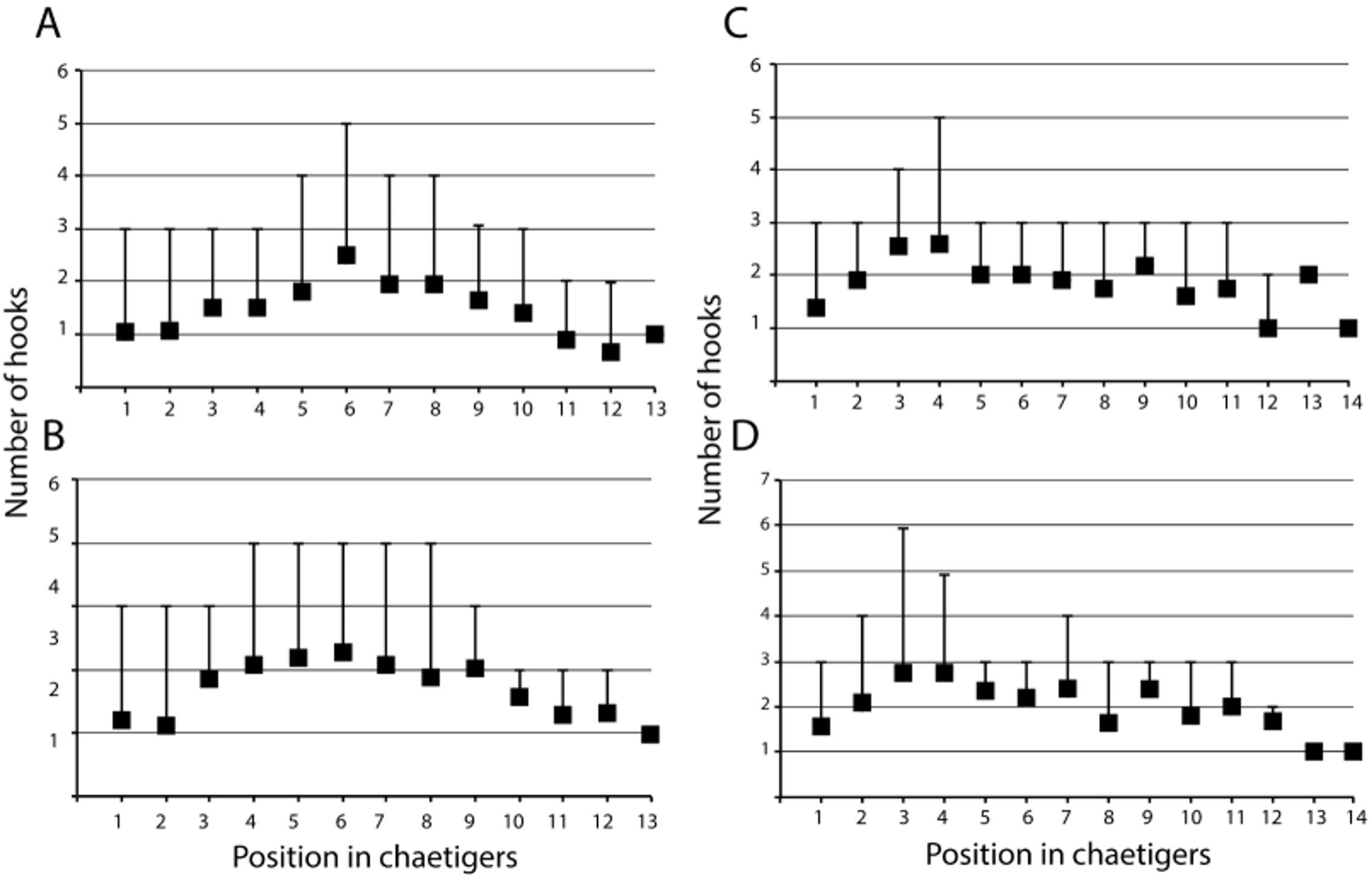
Chaetae all multidentate hooks with 3–5 teeth ( Figs 2 View FIGURE 2 C, 3D, 4D, F, G); proximal tooth enlarged and pointed down while others smaller and straighter ( Fig. 4 View FIGURE 4 F, G). Number of hooks per segment increasing to chaetiger 6 then decreasing to posterior end; from 1–4 (rarely 5) hooks per noto- or neuropodium. Shape of hooks similar throughout, short, thick and slightly curved; number of teeth not related to position along body or within fascicle; hooks with lesser or greater number of teeth randomly present along parapodia.
Pygidium elongated segment with dorsal anal aperture; anal cilia not observed ( Fig. 4 View FIGURE 4 E).
Remarks. See Table 1 View TABLE 1 and Discussion for comparison among species of Ctenodrilus . The number of teeth present in the hooks has been extensively used for species designation but is very variable. For instance, individuals of C. serratus from Massachusetts presented up to 5 teeth of similar shape and size ( Fig. 1 View FIGURE 1 A) while material from the Caribbean had a larger proximal tooth and up to three distal teeth ( Fig. 1 View FIGURE 1 C). C. pacificus sp. nov. possesses hooks with proximal tooth enlarged and pointed down and up to 4 distal teeth ( Figs. 2 View FIGURE 2 C, 3D, 4D, F, G). The number of hooks per segment and distribution along the body was different in Ctenodrilus pacificus sp. nov. and the Ctenodrilus serratus material examined from the North Atlantic Ocean. In the 20 examined specimens of Ctenodrilus pacificus sp. nov., the number of hooks per noto- and neuropodia increases from chaetiger 1 to 6 and then decreases to the end of the body ( Fig. 5 View FIGURE 5 A, B). In eleven individuals of Ctenodrilus serratus examined, the greatest number of hooks is present in earlier segments (chaetiger 3 or 4) and then decrease to the end of the body ( Fig. 5 View FIGURE 5 C, D). In both cases, average values were used and the same observation held true for individuals with 5–14 body segments. This needs to be further investigated with a greater number of specimens to evaluate if this character is size dependent.
The distribution of ventral cilia in the anterior end of Ctenodrilus pacificus sp. nov. seems to be different from all other Ctenodrilus species, if the illustrations provided are accurate. Cilia on ventral surface of anterior region is present until end of chaetiger 2 in C. serratus (see Fig. 1 View FIGURE 1 in Wilfert 1973), C. s. limulicolus (see Fig. 14a in Sudzuki & Sekiguchi 1972) and C. parvulus (see Fig. 1 View FIGURE 1 in Scharff 1887) and extending to anterior end of chaetiger 3 in Ctenodrilus pacificus sp. nov.
One paratype of Ctenodrilus paucidentatus was examined. Ben-Eliahu (1976) described this species as distinct from C. serratus based on the shape of the multidentate hooks with fewer teeth. The hooks in the paratype are as described and illustrated (Ben-Eliahu 1976; Fig. 4 View FIGURE 4 ) with a larger basal tooth and up to four smaller superior teeth. The validity of C. paucidentatus is questionable because it has been shown that the number of teeth in the multidentate hooks is very variable even within an individual and the paratype most likely belongs to Ctenodrilus serratus .
Etymology. The species name comes from the Latin pacificus , proposed by the occurrence of this species in the middle of the Pacific Ocean.
Distribution. This species has been collected in the north and south shores of Oahu, Hawaii.
Asexual reproduction. Several polychaete species can regenerate both anterior and posterior ends from fragments but few species present an organized fragmentation and regeneration pattern and use it as a normal feature of the life cycle ( Clark 1977). Asexual reproduction has been described for Ctenodrilus serratus and C. parvulus ( Scharff 1887; Schmidt 1857). The process has been demonstrated to start with the formation of new prostomia along the body usually producing between 3–5 stolons but Kennel (1882) has reported up to nine of them. Gelder & Palmer (1976) investigated the nervous system during paratomic fission of C. serratus . The prostomium of the still developing stolon individuals already presents the neuropile and circumesophageal connectives but the ventral nerve cords remain intact passing through the zone of fission and under the developing muscular proboscis. The authors showed that the recently detached stolons had already developed a central nervous system but no clear organization of the stomodeal innervation or circumanal loop. The digestive system also develops after separation.
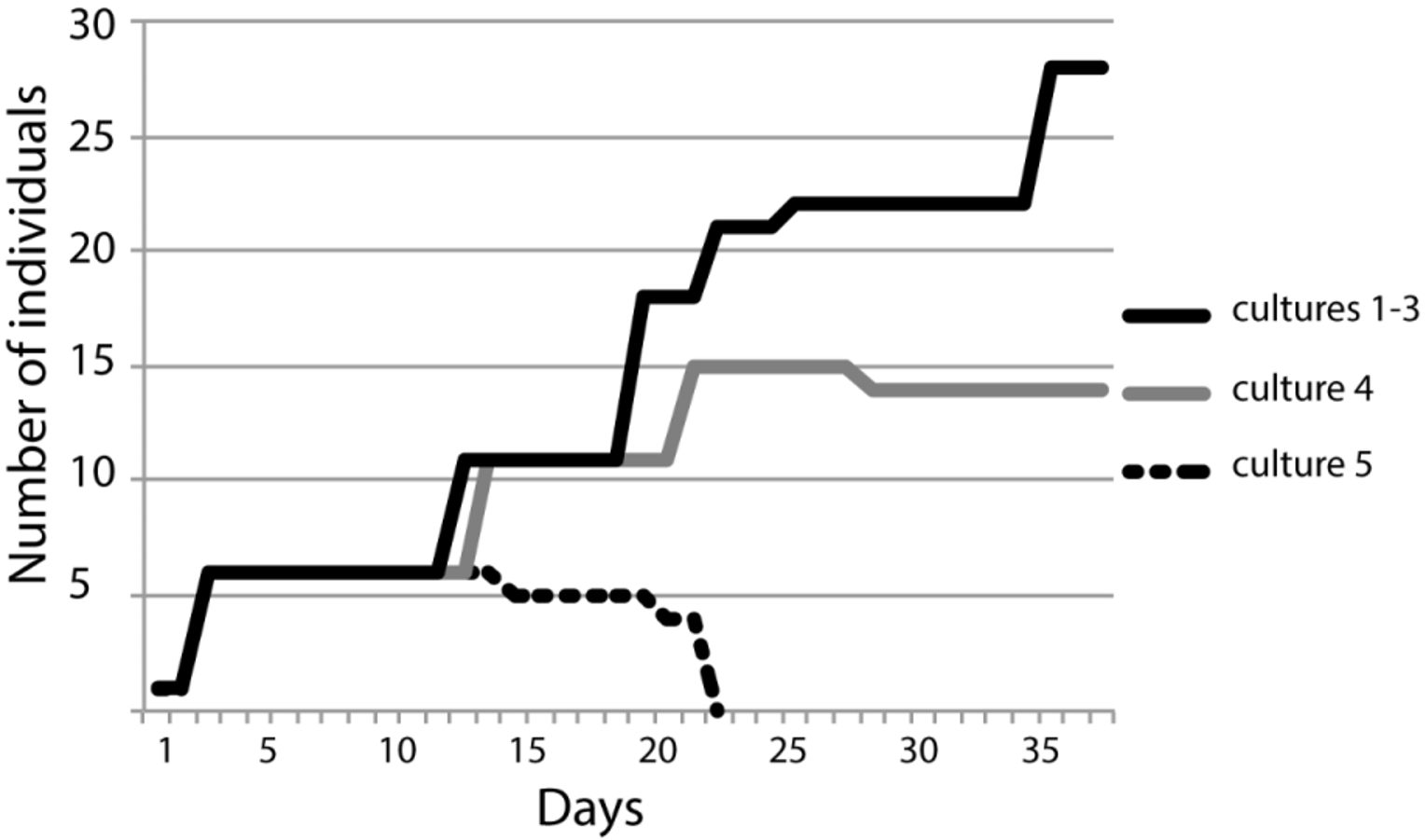
Five individuals showing well-demarked stolons were placed in separated containers and observed daily for a total of 37 days. All five trials started off with stock individuals showing five developing prostomia. Before the prostomia of the newly developing stolons start to appear, inter-segmental areas become clear of dark-pigmented oil cells making the segments appear well-separated ( Fig. 6 View FIGURE 6 A). A clear round region is also present and indicates the position of the pygidium ( Fig. 6 View FIGURE 6 A). Round cells were observed in several stock individuals at this stage, usually concentrated in the posterior end segments ( Fig. 2 View FIGURE 2 E). Zeppelin (1883) and Galvagni (1905) agreed that these cells may serve as storage for the stolons that are still unable to feed; however, these cells were not present in the developing stolons but on the posterior end fragment, casting doubt on this conclusion.
The developing stolons with clearly formed prostomia appear 24 hours later, but all lack nuchal organs ( Fig. 6 View FIGURE 6 A). The stolon individuals do not add new segments but the segment in which they are placed is now more elongated. Following this lengthening, the inter-segmental region and the digestive tract start to narrow. In less than 48 hours it is possible to see newly separated stolons with one long segment without mouth and gut disconnected from both anterior and posterior ends ( Fig. 6 View FIGURE 6 B). This is in agreement with observations by Scharff (1887) that from formation of new prostomia to separation of stolons takes up to 48 hours. Nuchal organs, heart body and nephridia are not fully developed at this stage. A group of cells is visible where the nuchal organs are expected to be and the region is now clear of pigment. Completely developed individuals with newly added segments are seen in less than 60 hours after the appearance of the buds ( Fig. 6 View FIGURE 6 C).
There were 28 clone individuals in three out of the five cultures, after 37 days (see Fig. 7 View FIGURE 7 ). Culture 5 crashed and all individuals died after 22 days. The anterior end of the recently divided organisms produces new individuals within less than 13 days. There was no control on the number of clones that were already producing new stolons. The number of clones produced per individual was not constant in each successive cloning event. Reish (1980) reported that a complete life cycle of Ctenodrilus serratus collected from Los Angeles Harbor takes approximately 14–21 days.
Parasites. Gregarine parasites (Apicomplexa) of the genus Selenidium were found in abundance in all individuals of Ctenodrilus pacificus sp. nov. examined in vivo. They were observed in the gut and intestine. Selenidium species have been reported to infect polychaetes of the Spionidae , Sabellidae and Serpulidae and more recently in Dodecaceria concharum ( Wakeman & Leander 2013) .
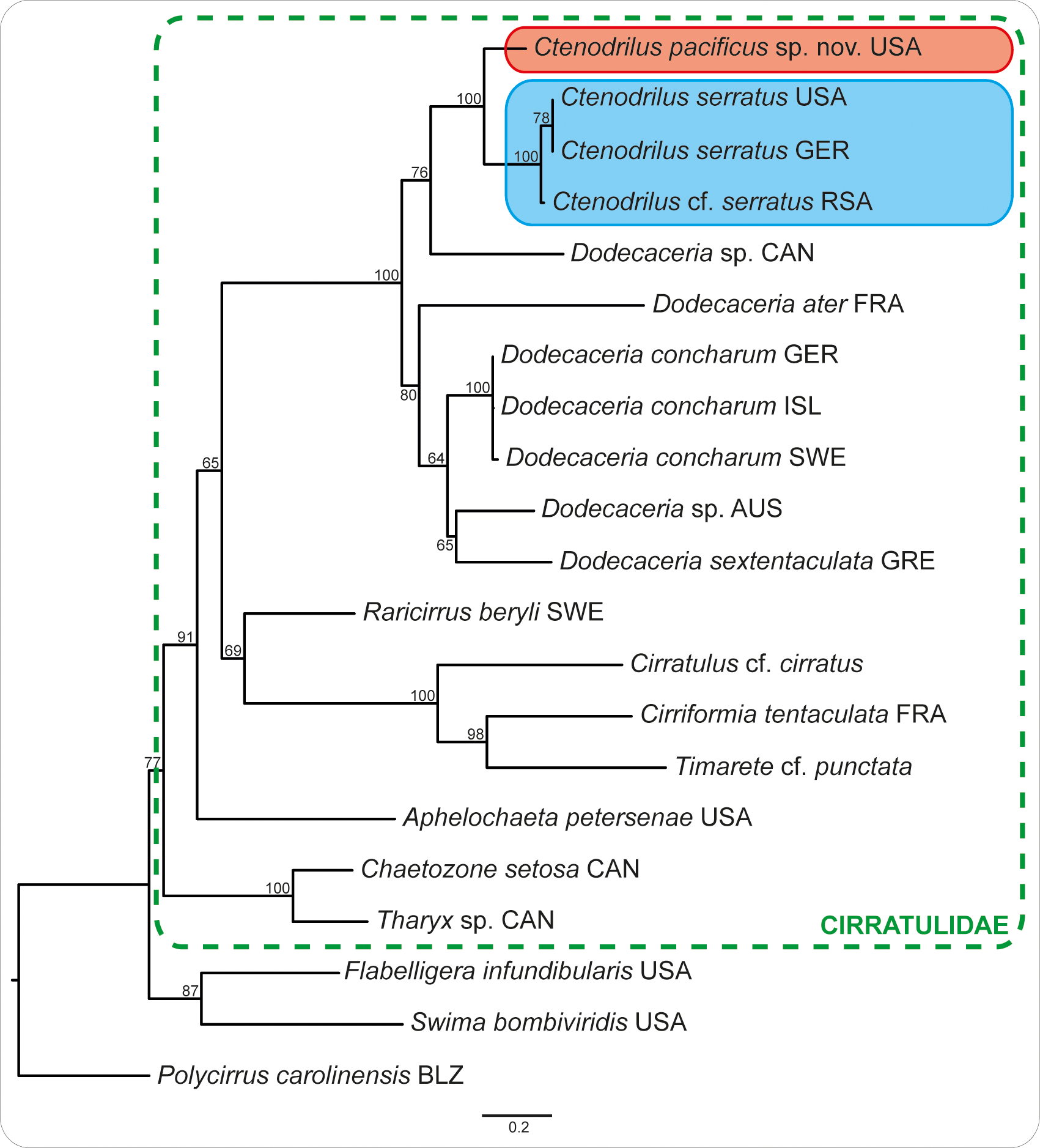
Phylogenetic results. Based on molecular data, Ctenodrilus pacificus sp. nov. is found to be a sister taxon to Ctenodrilus serratus including C. cf. serratus from Kleinzee, South Africa ( Fig. 8 View FIGURE 8 ). The K2P distances between both taxa are at least 10.9 % for 16S and 18.7 % for CO1 ( Table 3 View TABLE 3 ), supporting the hypothesis of both being distinct species. Together with Dodecaceria , Ctenodrilus constitutes a monophyletic group, but ingroup relationships remain questionable. While in the 16S analysis Ctenodrilus is nested within Dodecaceria , in the CO1 analysis both are resolved as reciprocal monophyletic groups (not shown). Additional molecular data for the genera Raphidrilus , Raricirrus and Aphropharynx will help elucidate the relationships between ctenodrilids and cirratulids but the affiliation of Ctenodrilus to the Cirratulidae is beyond question (see discussion in Weidhase et al. 2016).
1 CO
TABLE 3. Pairwise sequence distances (K 2 P) of analyzed Ctenodrilus.
| 16S | ||||
|---|---|---|---|---|
| Specimen | (1) | (2) | (3) | (4) |
| (1) Ctenodrilus pacificus sp. nov. | 15.5 % | 11.9 % | 10.9 % | |
| (2) Ctenodrilus serratus USA | - | 0.0 % | 0.3 % | |
| (3) Ctenodrilus serratus GER | 21.5 % | - | 0.6 % | |
| (4) Ctenodrilus cf. serratus RSA | 18.7 % | - | 4.1 % |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.