Syllis maganda, Martínez & Martín, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4834.2.5 |
|
publication LSID |
lsid:zoobank.org:pub:C6EC3841-60F7-4A99-AC88-D4FC6310CB83 |
|
DOI |
https://doi.org/10.5281/zenodo.4452287 |
|
persistent identifier |
https://treatment.plazi.org/id/A7D14743-8132-4A69-89A3-402ADF2FB1FA |
|
taxon LSID |
lsid:zoobank.org:act:A7D14743-8132-4A69-89A3-402ADF2FB1FA |
|
treatment provided by |
Plazi |
|
scientific name |
Syllis maganda |
| status |
sp. nov. |
Syllis maganda View in CoL n. sp.
Figures 2D View FIGURE 2 , 7 View FIGURE 7 , 8 View FIGURE 8 , 9 View FIGURE 9 , 10 View FIGURE 10 , 11 View FIGURE 11
LSID: urn:lsid:zoobank.org:act:A7D14743-8132-4A69-89A3-402ADF2FB1FA
Material examined. PHILIPPINES, Luzón, “Koala Point”, Balayan Bay, dead coral, 5–16 m depth, Dec 2010, coll. M.T. Aguado, P. Álvarez-Campos, C. Russell & G. San Martín, holotype ( MNCN 16.01 About MNCN /18678) and 2 paratypes ( MNCN 16.01 About MNCN /18679). Luzón, “House reef”, Balayan Bay , on hydrozoans, gorgonians ( Siphonogorgia sp.), ascidians ( Atriolum sp.) and dead coral, 2–4 m depth, Dec 2010, coll. M.T. Aguado, P. Álvarez-Campos, C. Russell & G. San Martín, 2 paratypes ( MNCN 16.01 About MNCN /18680). Luzón, “House reef”, Balayan Bay , dead coral, 2–3 m depth, Dec 2010, coll. M.T. Aguado, P. Álvarez-Campos, C. Russell & G. San Martín, 1 paratype ( MNCN 16.01 About MNCN /18681). “Sombrero Island”, Balayan Bay , dead coral, 2–3 m depth, Dec 2010, coll. M.T. Aguado, P. Álvarez-Campos, C. Russell & G. San Martín, 1 paratype ( MNCN 16.01 About MNCN /18682). “Sombrero Island”, Balayan Bay , dead coral, 1–2 m depth, Dec 2010, coll. M.T. Aguado, P. Álvarez-Campos, C. Russell & G. San Martín, 1 paratype ( MNCN 16.01 About MNCN /18683). “Mainif point”, between Balayan Bay and Batangas Bay, on dead coral and Halimeda sp., 1–2 m depth, Dec 2010, coll. M.T. Aguado, P. Álvarez-Campos, C. Russell & G. San Martín, 2 paratypes ( MNCN 16.01 About MNCN /18684). “Beatrice Point”, Sombrero Island , blue sponge, 2–3 m depth, Dec 2010, coll. M.T. Aguado, P. Álvarez-Campos, C. Russell & G. San Martín, 2 paratypes ( MNCN 16.01 About MNCN /18685). “Sepok Wall”, between Balayan Bay and Batangas Bay, dead coral, 6 m depth, Dec 2010, coll. M.T.Aguado, P. Álvarez-Campos, C. Russell & G. San Martín, 6 paratypes ( MNCN 16.01 About MNCN /18686), one for SEM (16.01/18687). “Sepok Wall”, between Balayan Bay and Batangas Bay, dead coral, 2–3 m depth, Dec 2010, coll. M.T. Aguado, P. Álvarez-Campos, C. Russell & G. San Martín, 4 paratypes ( MNCN 18688 About MNCN ) . EAST TIMOR, E of Atauro Island, 08°14’30”S 125°36’49”E, Inner reef, reef slope, branching coral matrix with epiphytic algae and sponges, 14 m depth, Sep 2012, coll. L.E. Hughes, 1 paratype ( AM W.45651) GoogleMaps .
Additional material. PHILIPPINES. Luzón island, House reef, Balayan Bay , dead coral, 2-3 m depth, 7/12/2010, two specimens ( MNCN 16.01 About MNCN /18689). Luzón Island , House reef, Balayan Bay , dead coral, 2-3 m depth, 12/2010, four specimens ( MNCN 16.01 About MNCN /18690). Luzón Island , House reef, Balayan Bay , dead coral, 2-4 m depth, 4/12/2010, 1 specimen ( MNCN 16.01 About MNCN /18691). Luzón Island , Koala Point, Balayan Bay , dead coral, 5-16 m depth, 5/12/2010, two specimens ( MNCN 16.01 About MNCN /18692). Mainif point, between Balayan Bay and Batangas Bay, inside Xetospongia , 8/12/2010, 8/12/2010, 1 specimen ( MNCN 16.01 About MNCN /18693) .
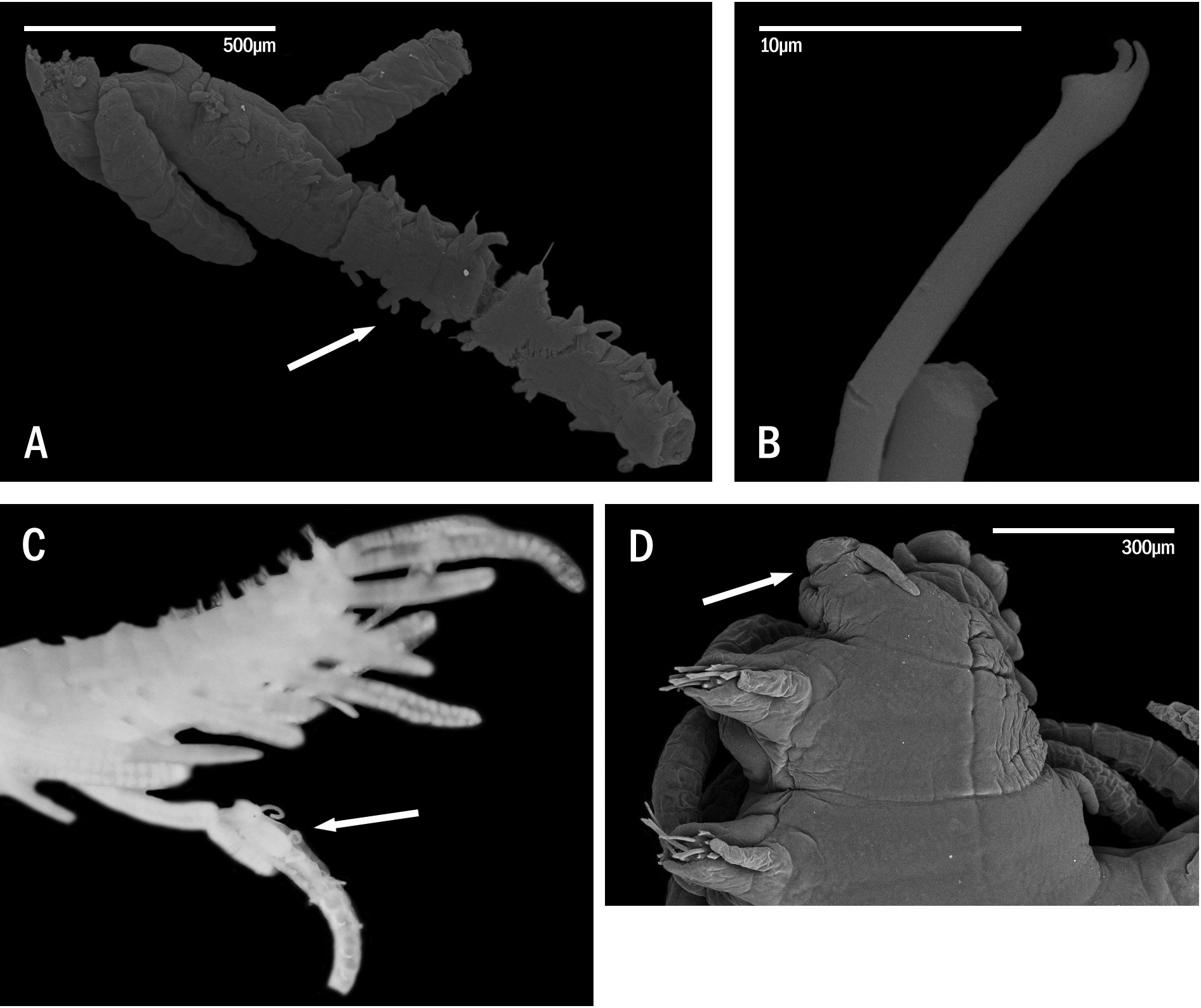
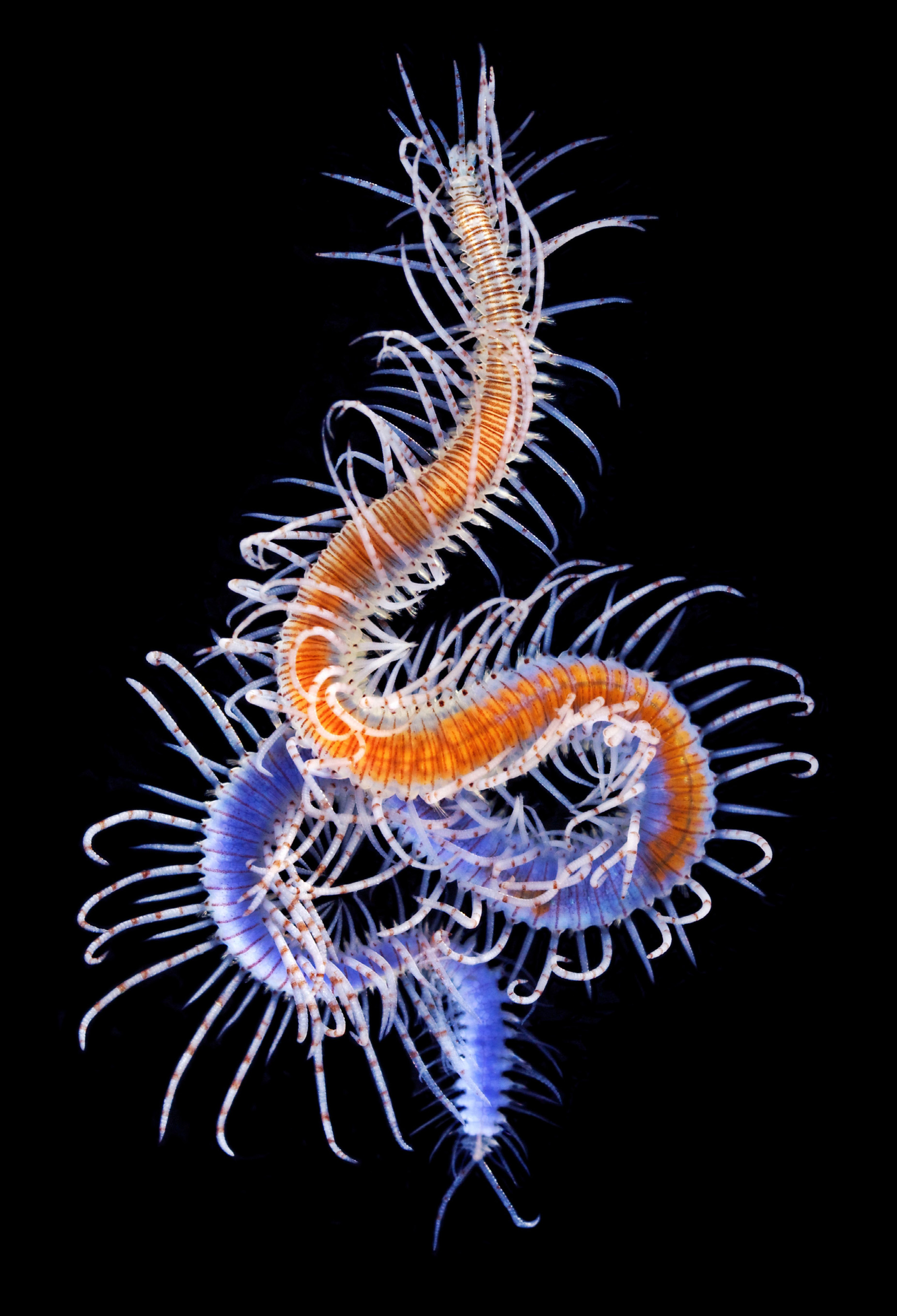
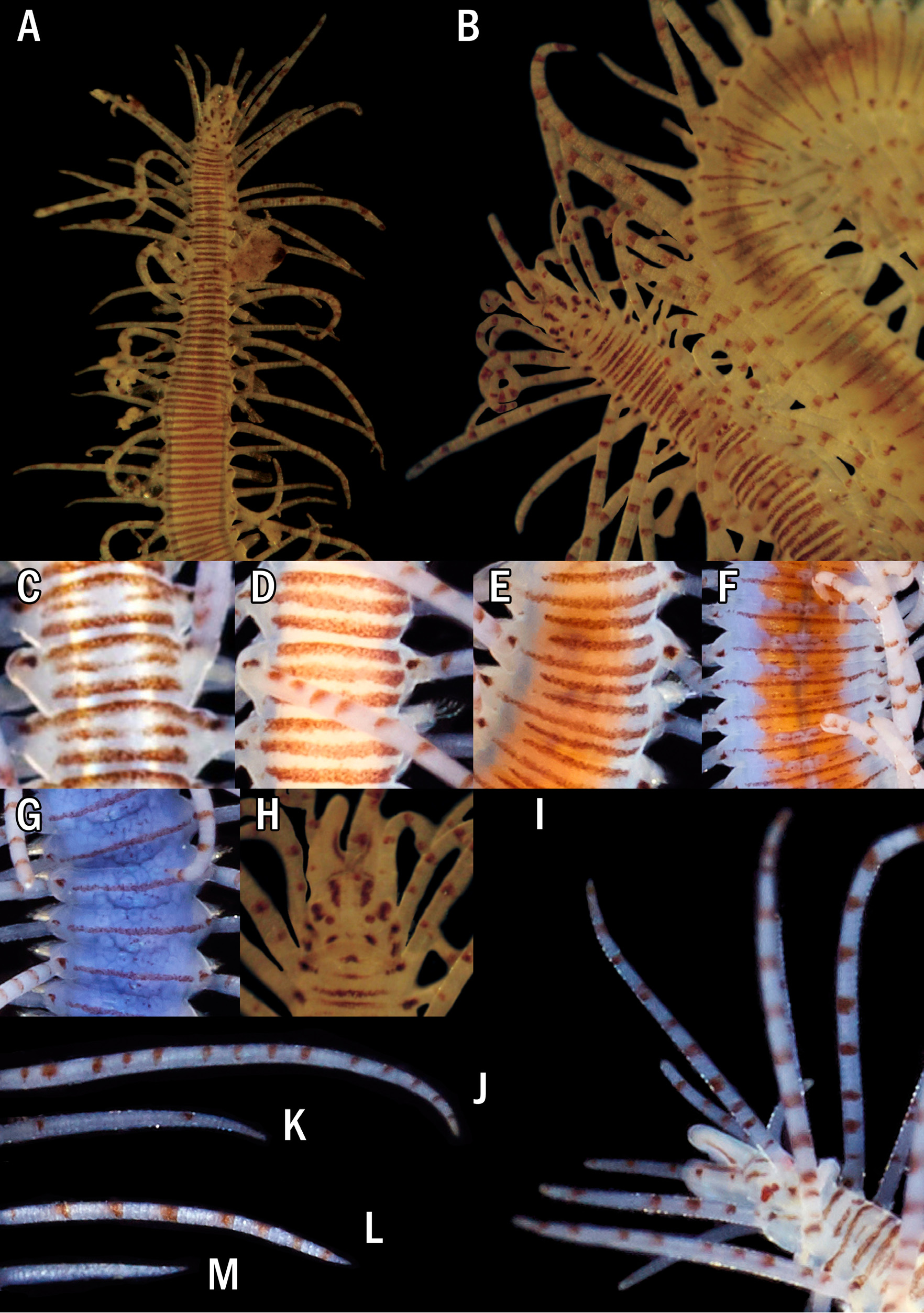
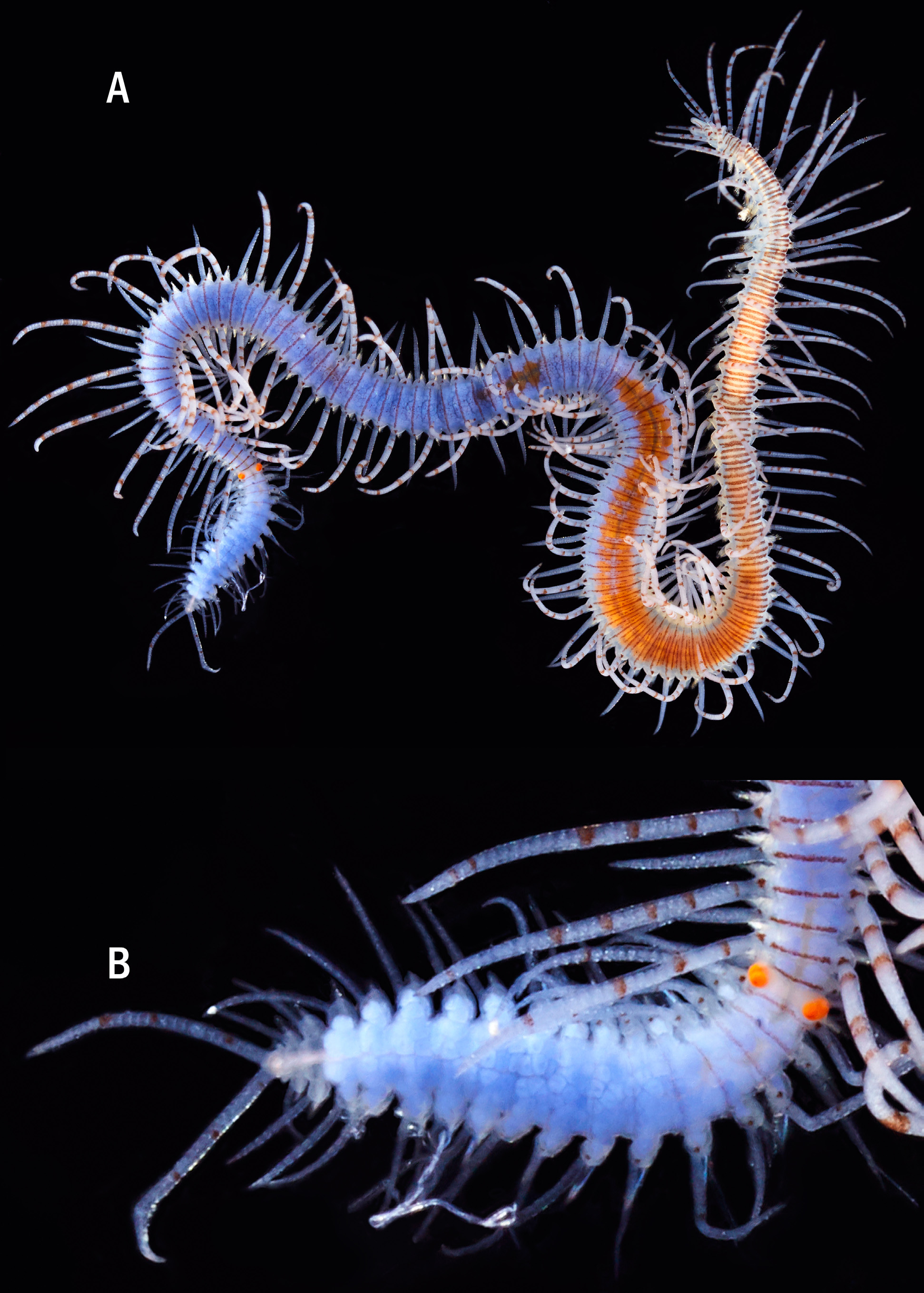
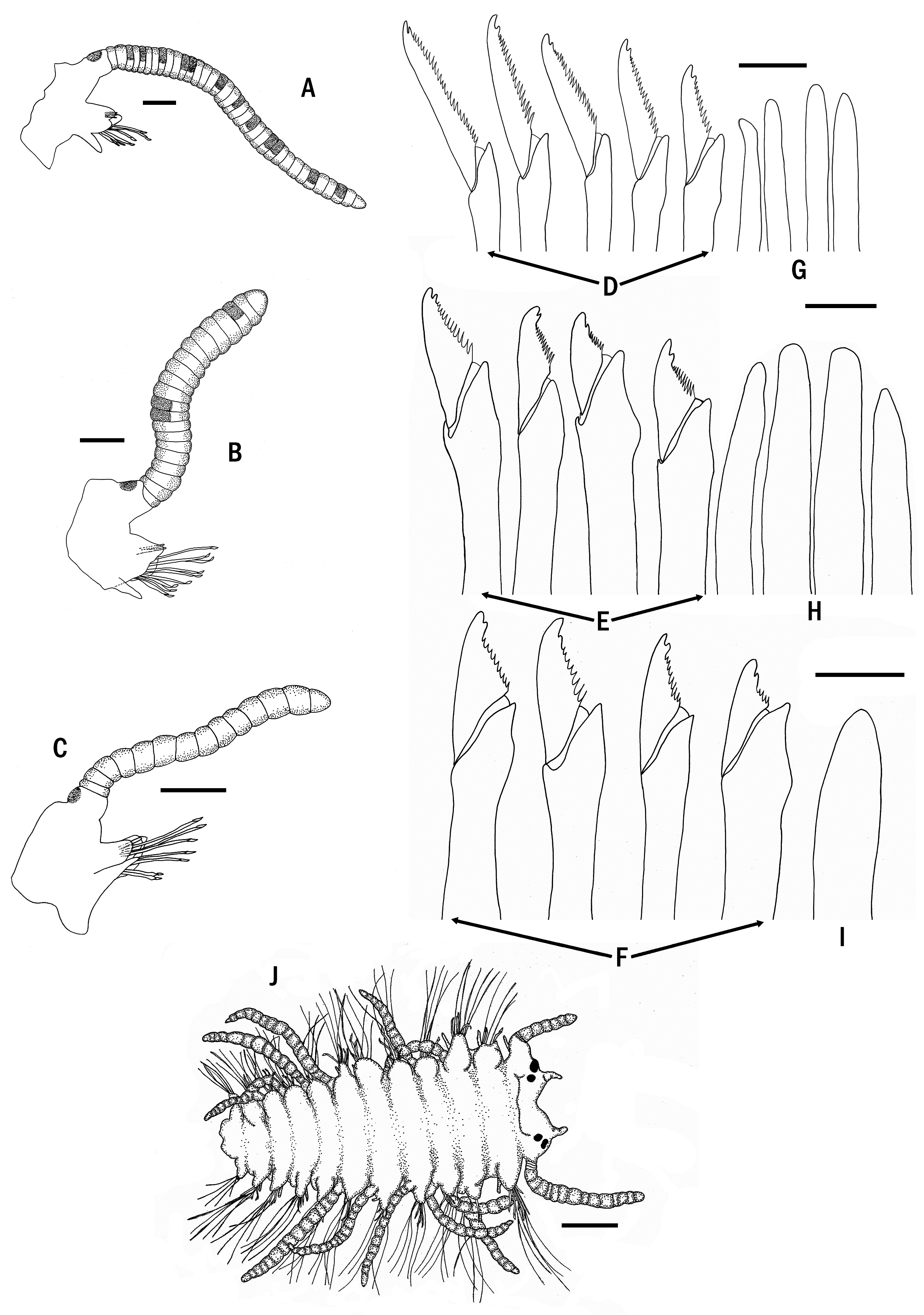
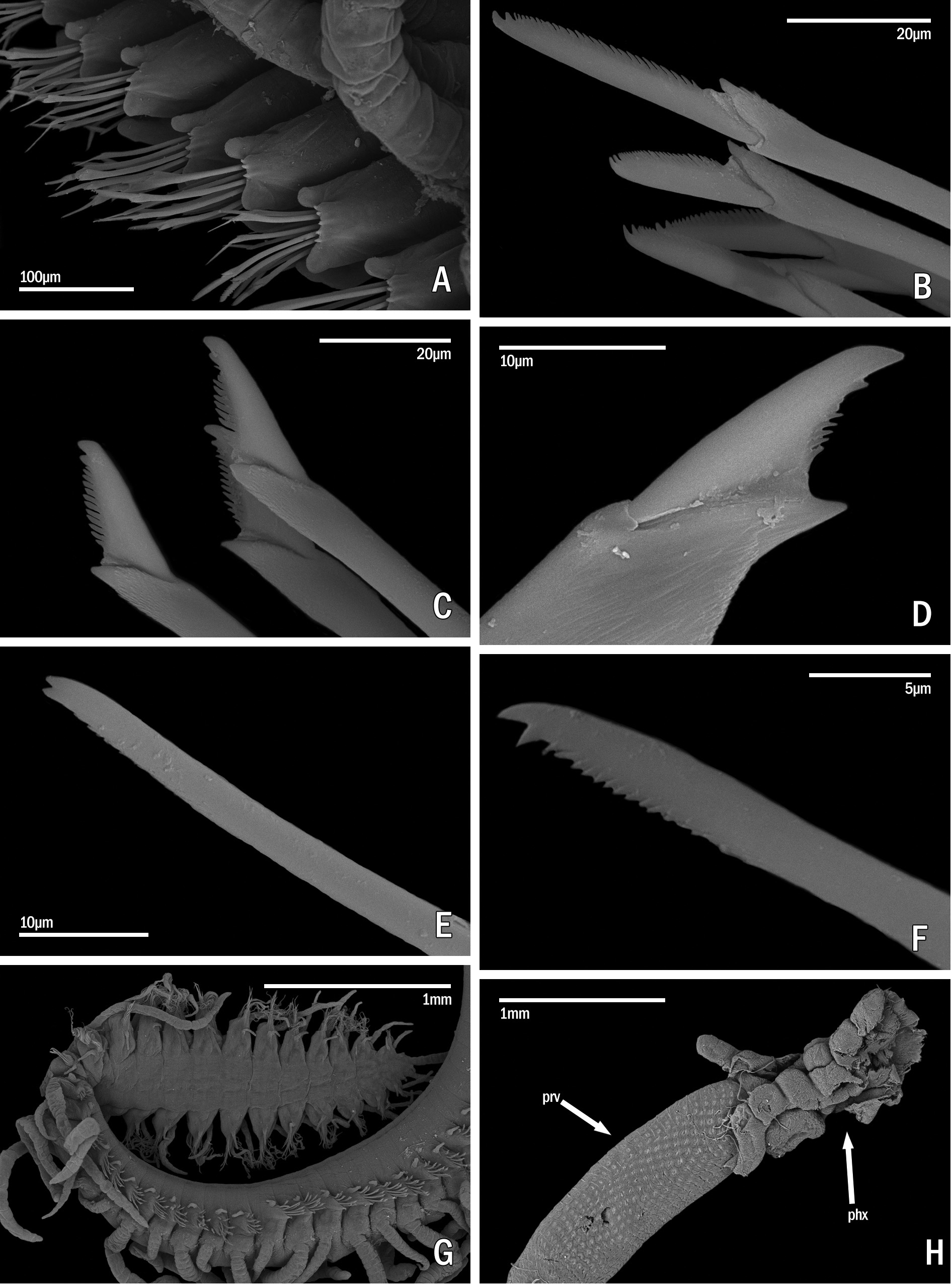
Description. Holotype ( Fig. 8A, B, H View FIGURE 8 ) complete specimen, 36 mm long, 1.5 mm wide, with 93 chaetigers. Largest complete paratype, 37 mm long, 1.5 mm wide, with 131 chaetigers. Live specimens brightly coloured ( Figs 7 View FIGURE 7 , 8 View FIGURE 8 C–G, I–M, 9A, B); only red lines and spots retained in fixed specimens ( Fig. 8A, B, H View FIGURE 8 ), losing the orange and blue colourations present in the dorsum and cirri. Body elongated, with orange dorsum, more intense in centre of segments, somewhat pale on anterior body quarter, more intense on next quarter, paler again on two posterior quarters, which become intense blue-violet ( Figs 7 View FIGURE 7 , 9A View FIGURE 9 ) due to intracoelomic oocytes ( Fig. 8G View FIGURE 8 ); some midbody segments with middle orange colour and blue laterals ( Fig. 8F View FIGURE 8 ); most anterior segments with two horizontal continuous red lines, one anterior and one posterior, and a discontinuous intermediate, central line ( Fig. 8C View FIGURE 8 ); most anterior midbody segments with three continuous lines ( Fig. 8D View FIGURE 8 ), anterior and posterior lines shorter than central one at midbody ( Fig. 8E View FIGURE 8 ), becoming progressively smaller and less marked ( Fig. 8F View FIGURE 8 ); posterior segments with only a continuous central line ( Fig. 8G View FIGURE 8 ). Some specimens with Dicerous stolons ( Figs 9B View FIGURE 9 , 10J View FIGURE 10 , 11G View FIGURE 11 ) still attached, either lacking red lines or only having pale ones on most anterior segments. Dark red rounded spots at bases of dorsal cirri ( Figs 8 View FIGURE 8 C–G, 10A–C), on prostomium and on palps ( Fig. 8H, I View FIGURE 8 ); usually four spots on prostomium, arranged regularly, anterior pair elongate and oblique, posterior pair round; sometimes with some smaller spots, particularly between eyes, two elongated spots on inner side of palps, and a small spot, sometimes very faint on peristomium ( Fig. 8H View FIGURE 8 ). Antennae, tentacular cirri and dorsal cirri whitish to yellowish, with one reddish spot on a certain number of articles (5–8 articles in every long dorsal cirrus; 1–2 on short cirri) ( Fig. 8I View FIGURE 8 ); anterior dorsal cirri ( Fig. 8J, K View FIGURE 8 ) more pigmented than posterior ones ( Fig. 8L, M View FIGURE 8 ); spots disappearing on most posterior short cirri ( Fig. 8M View FIGURE 8 ). Long anterior cirri with eight or more spots ( Fig. 8J View FIGURE 8 ), long posterior ones with 7–8 ( Fig. 8L View FIGURE 8 ). Prostomium semicircular, with four red eyes in trapezoidal arrangement ( Fig. 8H View FIGURE 8 ); eyespots not seen. Palps robust, elongated, somewhat longer than prostomium ( Fig. 8H, I View FIGURE 8 ). Median antenna arising from posterior part of prostomium, between posterior eyes, approximately twice or twice and a half longer than combined length of prostomium and palps, with 25–50 articles ( Fig. 8I View FIGURE 8 ); lateral antennae half or one third shorter than median antennae, with 23–30 articles ( Fig. 8I View FIGURE 8 ). Peristomium somewhat shorter than subsequent segments, forming a small hood covering posterior part of prostomium ( Fig. 8H View FIGURE 8 ). Dorsal tentacular cirri as long as median antenna, with 25-50 articles ( Fig. 8I View FIGURE 8 ); ventral tentacular cirri as long as lateral antennae, with approximately same number of articles ( Fig. 8I View FIGURE 8 ). Dorsal cirri somewhat thick at bases, becoming progressively thinner distally, with pointed tips ( Figs 8 View FIGURE 8 J–M, 10A–C); distinct alternating long (more than three times longer than body width) ( Figs 7 View FIGURE 7 , 9A View FIGURE 9 ) and somewhat thinner short cirri ( Fig. 8K, M View FIGURE 8 ); short dorsal cirri ( Fig. 8K, M View FIGURE 8 ) less pigmented than long ones ( Fig. 8J, L View FIGURE 8 ). Dorsal cirri of chaetigers 1, 4 and 6 longer than remaining ones; dorsal cirri of midbody long (60 articles) and short (30–40). Dorsal cirri of stolons all similar, short and thin ( Figs 9B View FIGURE 9 , 10J View FIGURE 10 , 11G View FIGURE 11 ). Parapodial lobes conical, with two small lips, anterior digitiform and posterior round, shorter and a small round middorsal lobe ( Figs 10 View FIGURE 10 A–C, 11A). Ventral cirri thin, digitiform, somewhat shorter than parapodial lobes ( Fig. 10 View FIGURE 10 A–C). Compound chaetae heterogomph falcigers, with relatively short, bidentate blades; proximal teeth distinctly smaller and thinner than distal ones, with straight, short marginal spines, slightly longer and thinner on anterior chaetae; anterior chaetae have both teeth slightly more separated from each other than those of midbody chaetae, being even closer each other in posterior ones; blades gradually decreasing in length towards posterior segments ( Figs 10 View FIGURE 10 D–F, 11B–D). Anterior parapodia ( Figs 10A View FIGURE 10 , 11A View FIGURE 11 ) with 13–14 compound chaetae with marked dorso-ventral gradation, blades about 60 µm long dorsally, 35 µm ventrally ( Figs 10D View FIGURE 10 , 11B View FIGURE 11 ); midbody parapodia ( Fig. 10B View FIGURE 10 ) with about 10–11 compound chaetae; articles distinctly shorter and wider than those of anterior segments, with less marked dorsoventral gradation, 40- 30 µm long ( Figs 10E View FIGURE 10 , 11C View FIGURE 11 ); posterior parapodia ( Fig. 10C View FIGURE 10 ) with 7–8 compound chaetae, similar in shape and size to those of midbody, with shorter marginal spines ( Figs 10F View FIGURE 10 , 11D View FIGURE 11 ). Dorsal simple capillary chaetae on posterior parapodia, thin, finely bidentate, both teeth small, equal in size, close each other, with a rounded tip, with very short marginal spines ( Fig. 11E View FIGURE 11 ). Ventral simple capillary chaetae on most posterior parapodia, somewhat thicker than dorsal ones, bidentate, both teeth large, similar in size, well spaced each other, pointed, with short marginal spines ( Fig. 11F View FIGURE 11 ). Anterior and midbody parapodia with four aciculae, three with blunt tips and one smaller with slightly oblique tip ( Fig. 10G, H View FIGURE 10 ); number of aciculae per parapodium decreasing progressively posteriorly to one in posterior parapodia, with acute and distally blunt tips ( Fig. 10I View FIGURE 10 ). Pharynx ( Fig. 11H View FIGURE 11 , phx) extending along 12 segments ( Fig. 8A View FIGURE 8 ); pharyngeal tooth on anterior margin, elongated, conical, sharp. Proventricle ( Fig. 11H View FIGURE 11 , prv) somewhat shorter and wider than pharynx, extending along six segments, with about 33 muscle cell rows. Pygidium short, with two long anal cirri, with 33 articles, similar in colour to posterior dorsal cirri ( Figs 7 View FIGURE 7 , 9A, B View FIGURE 9 ).
Remarks. Specimens photographed by Alexander Semenov and one Philippine paratype showed Chaetosyllis (Dicerous) stolons (i.e., with two dorsal and two ventral eyes, and two small articulated antennae) ( Figs 9B View FIGURE 9 , 10J View FIGURE 10 , 11G View FIGURE 11 ). Stolons are relatively small relative to specimen’s size (13–16 chaetigerous segments). Additionally, a specimen had a regenerated head ( Fig. 2D View FIGURE 2 , arrow).
Syllis maganda n. sp. is conspicuous because of its special and motley colour pattern, which differs from all other specie of Syllis having striking colourations, as well as for having a large size body, whip-shaped dorsal cirri composed of numerous small articles with a marked alternation in size and chaetae with relatively short and wide blades, except those of anterior-most chaetae, bidentate, with proximal tooth clearly smaller and thinner than distal one, both teeth becoming more separated from each other posteriorly. For instance, Syllis variegata Grube, 1860 , Syllis ferrani Alós & San Martín, 1987 and Syllis columbretensis Campoy, 1982 , from the Iberian Coast, show dorsal a ∞-shaped figure on each segment, but also more strongly bidentate chaetae ( San Martín 2003). Syllis alosae San Martín, 1992 , from Cuba, has a similar colour pattern than these aforementioned species, but more strongly bidentate chaetae, including some pseudospingers (chaeta with elongated article) on each fascicle ( San Martín 1992). Syllis unzima Simon, San Martín & Robinson, 2014 , from South Africa, has transversal segmental stripes but with a simpler pattern and, furthermore, it is viviparous ( S. maganda n. sp. reproduces by stolons), has almost unidentate chaetae and the pharyngeal tooth is located slightly behind the anterior margin of pharynx ( Simon et al. 2014). Syllis crassicirrata ( Treadwell, 1925) sharing a marked colour pattern and, partially, its geographical distribution (i.e., widely distributed in the Indo-Pacific). However, this species seemed to show a great variety of colourations which, in fact, could be an actually hiding a species-complex ( Ba-Akdah et al. 2018). Some pattern incude strong pigmentation, even with spots in dorsal cirri with, as in S. maganda n. sp. However, their dorsal cirri are relatively shorter and thicker and all similar in length, the chaetae are more strongly bidentate (especially the posterior ones), and the posterior aciculae are straight and extend out from the parapodial lobes ( Álvarez-Campos et al. 2015a; Ba-Akdah et al. 2018). Syllis ehlersioides Imajima, 1966 , from Japan, have similar long cirri and a noticeable colour pattern, although differs in consisting in wide transverse bands of chocolate colour, and the chaetae are almost unidentate and the aciculae tips are round ( Imajima 1966a).
Etymology. The specific name refers to its colouration, as maganda means “gorgeous and beautiful” in Tagalog. In addition to East Timor, this new species is also present in the Philippines where Tagalog is spoken. Therefore, it seems appropriate that the etymology of this new species has a meaning in this language.
Habitat. In amongst dead corals, with hydrozoans, gorgonians ( Siphonogorgia sp.), ascidians ( Atriolum sp.), and green algae ( Halimeda sp.) as epibionts; on a matrix of branched coral with epiphyte algae and sponges.
Distribution. Only known from the type locality of the Philippines, Australia (Lizard Island) and East Timor.
| AM |
Australian Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |